Question
In: Chemistry
Draw the organic product for the following reaction. Omit any inorganic byproducts or ions.
Solutions
Expert Solution
Concepts and reason
The concept used to solve this question is to use the functioning of the given reagent \(\operatorname{LiAlH}_{4}\). It is a reducing agent and is used in the reduction of a wide variety of functional groups. The given starting material is an amide, so the product formed by the reduction of amide in the presence of \(\operatorname{LiAlH}_{4}\) is the answer.
Fundamentals
Organic chemistry is a chemistry branch that deals with the synthesis of scaffolds using different reagents by taking a specific synthetic route. Every reagent has a specific application that can be used in the synthesis for the functional group transformation. The functional group in the compounds decides the reactivity of the compounds. The given reagent is \(\operatorname{LiAlH}_{4}\). The systematic name of this reagent is lithium aluminum hydride. The structure of \(\operatorname{liAlH}_{4}\) is as follows:
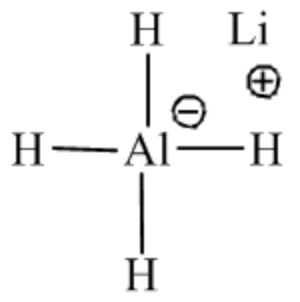
From the name itself, it is clear that it consists of a hydride ion, ready to attack the electron-deficient site of the compounds. So it acts as a reducing agent. It is used to reduce a wide variety of functional groups like carboxylic acid and their derivatives, carbonyl compounds, nitro compounds, nitrile compounds, and so on. The reactions of carboxylic acid and their derivatives with \(\operatorname{LiAlH}_{4}\) are shown below.
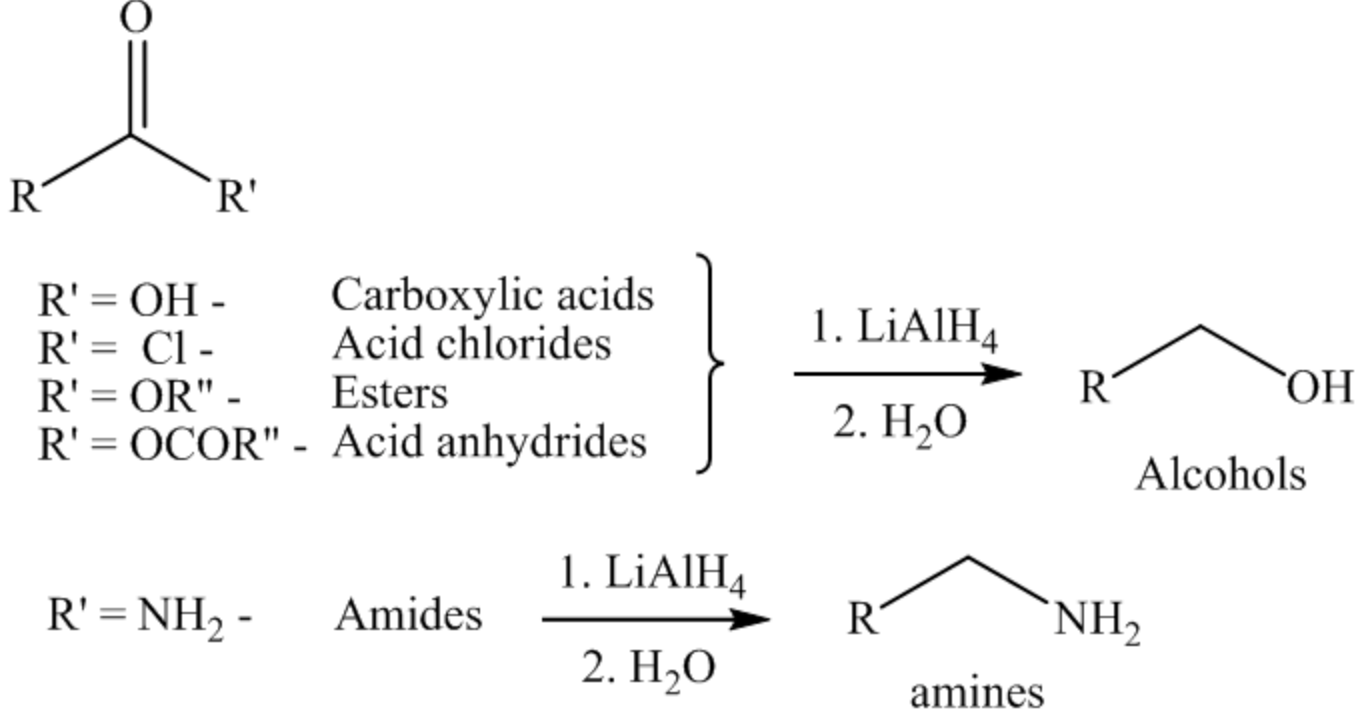
Hence, the formation of product in the LiAlH \(_{4}\) reaction depends on the functional group present on the starting material.
The structure of the starting material is shown below.
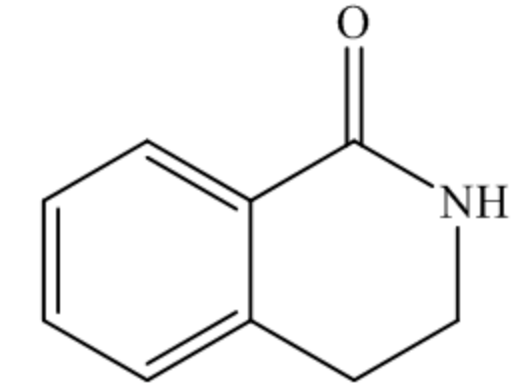
In the above structure, the carbon atom of the C=O bond is bonded to a nitrogen atom, so it is an amide bond. Thus, the given compound is a cyclic amide, which is also called lactam.
Therefore, the functional group present in the starting material is the “amide” functional group.
To write the reaction's product, one should know the functional group present in the starting material. From the structure of the starting material, it is observed that an amide functional group is present. So, the given compound is a cyclic amide.
The given starting material is an aromatic lactam, and it consists of an amide functional group. According to reactions of \(\operatorname{LiAlH}_{4}\) with the carboxylic acid derivatives, when an amide is treated with \(\operatorname{LiAlH}_{4},\) the carbonyl bond \((\mathrm{C}=\mathrm{O})\) of amide is reduced to \(\mathrm{CH}_{2}\) group and forms an amine as product. Therefore, the given reaction can be written as follows:
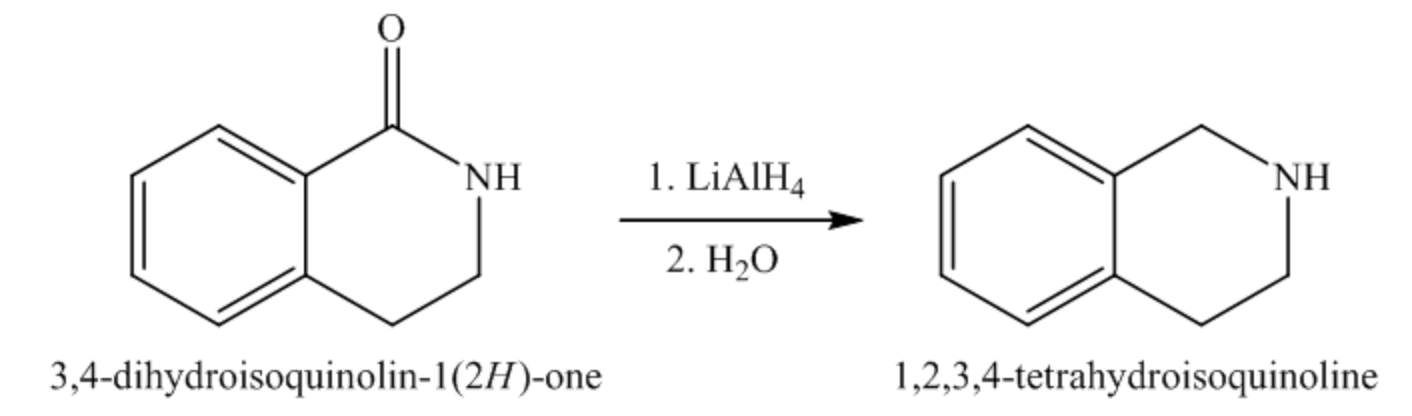
Thus, the organic product of the given reaction is shown below.
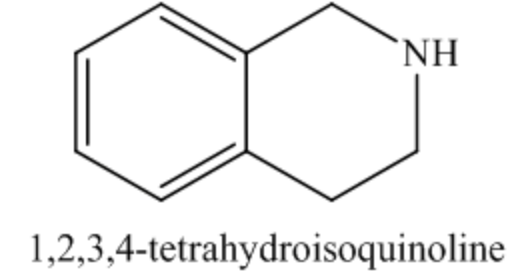
The functional group present in the starting material is an amide functional group. The reagent given in the reaction is \(\operatorname{LiAlH}_{4}\). \(\operatorname{liAlH}_{4}\) is a reducing agent, so it reduces the amide functional group into an amine. Thus, the cyclic amide on treatment with LiAlH \(_{4}\) undergoes reduction followed by hydrolysis gives cyclic amine as the product.
The organic product of the given reaction is as follows:
Related Solutions
Draw the organic product in each of the following reactions. Include formal charges, if applicable. Omit any inorganic byproducts or ions.
Draw the structures of organic compounds A and B. Omit all of the byproducts.
For the following SN2 reaction, draw the organic and inorganic products of the reaction, and identify...
For the following SN2 reaction, draw the organic and inorganic products of the reaction, and identify the nucleophile, substrate, and leaving group.
For the following SN2 reaction, draw the organic and inorganic products of the reaction, and identify the nucleophile, substrate, and leaving group.
Draw the major organic product of the reaction shown below.Draw the major organic product of...
Draw the structure of the organic product of each reaction in the following two-step synthesis.
Draw the organic product (if any) expected from the following reaction: (include all hydrogen atoms) CH3CH2CH2OH + K2Cr2O7(aq) H2SO4
Draw the organic products formed in the following reaction.
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago