Question
In: Chemistry
Draw the structures of organic compounds A and B. Omit all of the byproducts.
Draw the structures of organic compounds A and B. Omit all of the byproducts. acetaldehyde H2O
Solutions
Expert Solution
Concepts and reason
This problem is based on the concept of Grignard reagent reactivity. The grignard reagent is a very good nucleophile in the case of carbonyl compounds. It is made up of the reaction of haloalkane with Mg in the presence of ether. It attacks carbonyl compounds followed by the treatment with water and forms corresponding alcohol.
Fundamentals
In order to predict the reactant from the product, the reaction should be written backward (from product to reactant).
The reaction is given below:
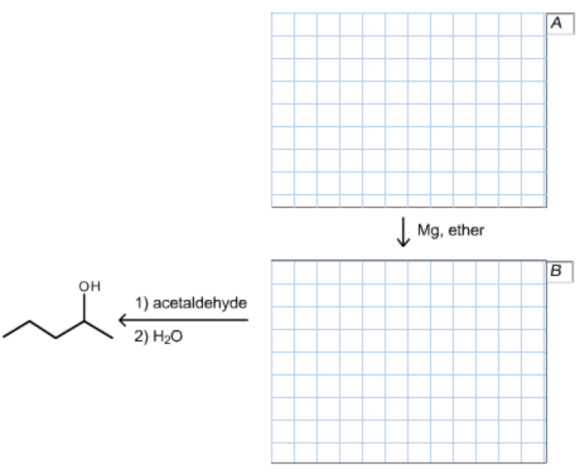
The product contains five carbons of which two carbons came from acetaldehyde and three carbons came from a nucleophile that is propyl magnesium bromide.
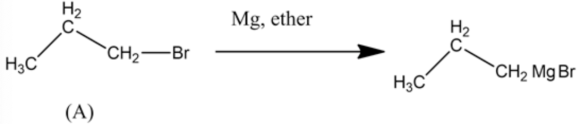
The first reaction of synthesis is given below:
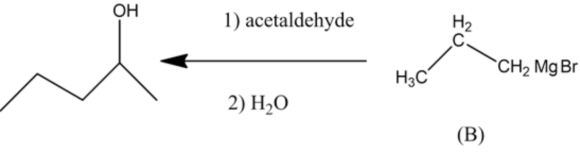
The structures of compounds \(A\) and \(B\) are as follow:
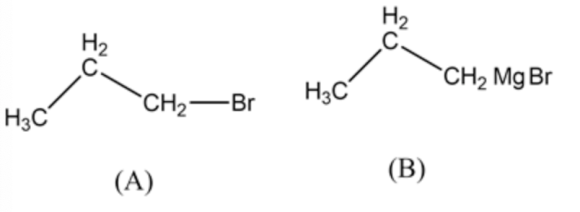
In this synthesis, propyl bromide molecule undergoes a chemical reaction with \(\mathrm{Mg}\) in the presence of ether in order to form Grignard reagent which on reaction with acetaldehyde followed by the treatment of water forms alcohol which pentan-2-ol.
Related Solutions
Draw the organic product for the following reaction. Omit any inorganic byproducts or ions.
Draw the structures of organic compounds A and B. Indicate stereochemistry where applicable.
Draw the organic product in each of the following reactions. Include formal charges, if applicable. Omit any inorganic byproducts or ions.
Draw Lewis structures for the following compounds.
what are the names and structures of the major organic compounds anticipated to be obtained in...
Starting with appropriate unlabeled organic compounds, show syntheses of each of the following: (a) Draw the...
Draw the structures of the organic products in each reaction of the following two-step synthesis.
Draw Lewis structures for each of the following compounds. In each case, specify the number of...
Draw resonance structures. Do not show ion charges in your drawings. a)Draw all resonance structures for...
Draw the condensed and Lewis structures for each of the following compounds (include lone pairs where...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
- For this assignment you will write a program with multiple functions that will generate and save...
- How many grays is this?Part A A dose of 4.7 Sv of γ rays in a...
- how to operate a business?
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago