Question
In: Chemistry
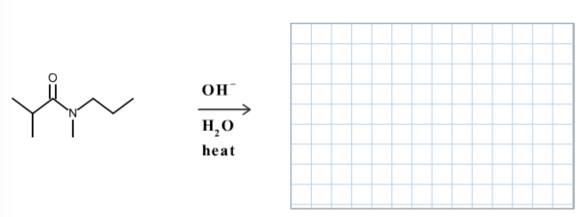
Draw the organic products formed in the following reaction.
Draw the organic products formed in the following reaction.
Solutions
Expert Solution
Concepts and reason
Amide hydrolysis is a process where an amide is hydrolyzed and forms a carboxylate ion in the presence of a base. In the presence of an acid, amide hydrolysis ends up with carboxylic acid and an ammonia derivative. Amide hydrolysis follows the mechanism of ester hydrolysis under basic conditions.
Fundamentals
- Amide hydrolysis under basic media produces the carboxylate ion and ammonia derivative. First, the base attacks the positively charged carbon atom in the carbonyl group of amide and forms oxy anion. Then, the oxy anion stabilizes to form carboxylic acid by giving the amine derivative. Under basic conditions, the carboxylic acid formed loses the proton to form a carboxylate ion.
- General amide hydrolysis under basic conditions
Given amide is shown below.
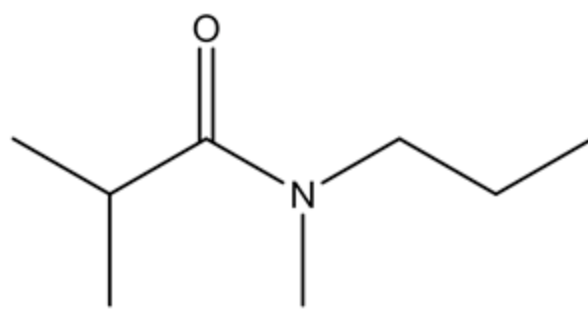
Base attack on the carbonyl group of the amide
Oxy anion is formed during the attack of the base on a carbonyl group of amide. The base attacks the partially positive carbon atom of the carbonyl group and produces the oxyanion, where the negative charge lies on the more electronegative oxygen atom.
Oxyanion
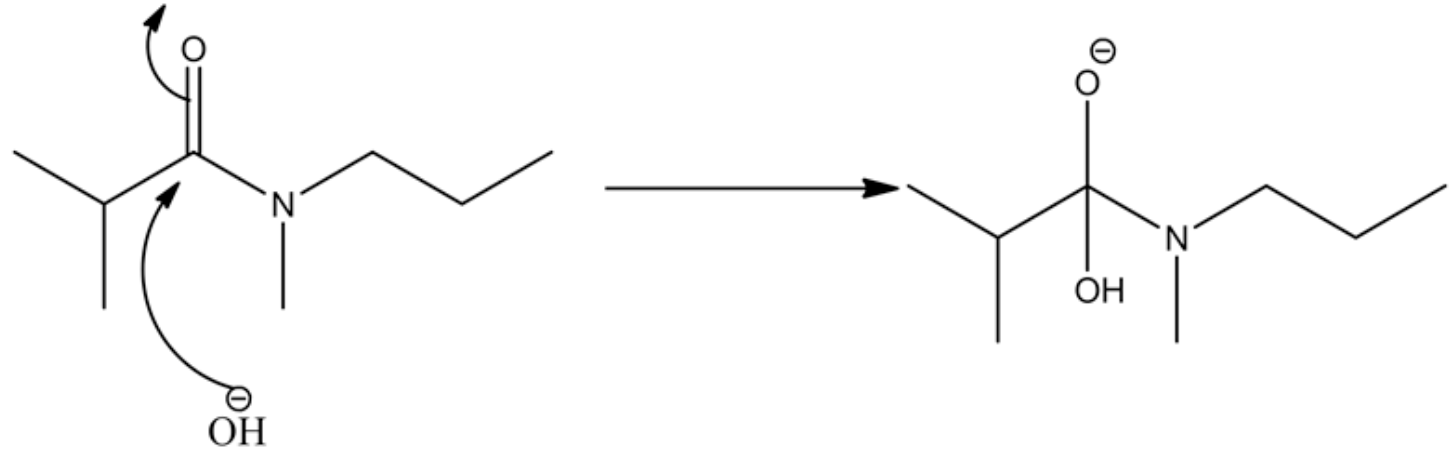
Stabilization of oxyanion
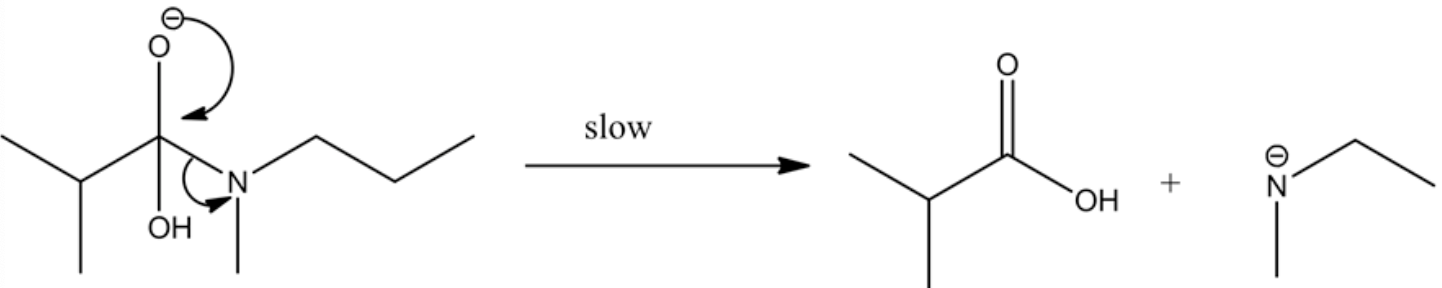
Stabilization of oxyanion to carboxylic acid is a slow step process, whereas carboxylic acid to carboxylate conversion in the presence of a base is very rapid.
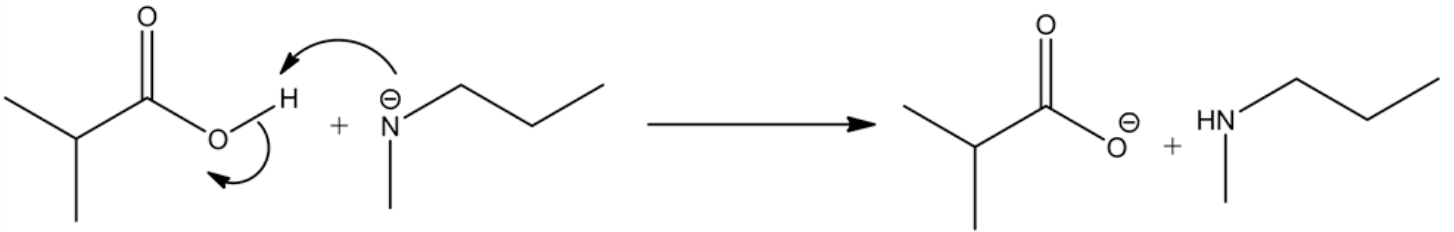
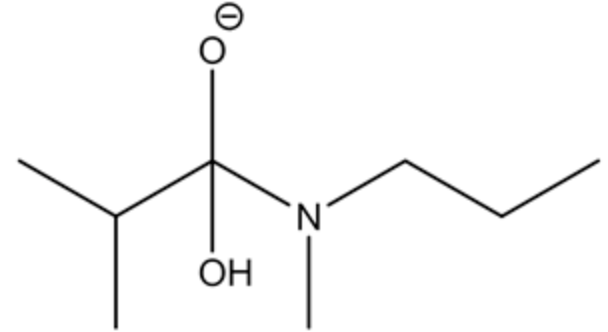
The complete reaction
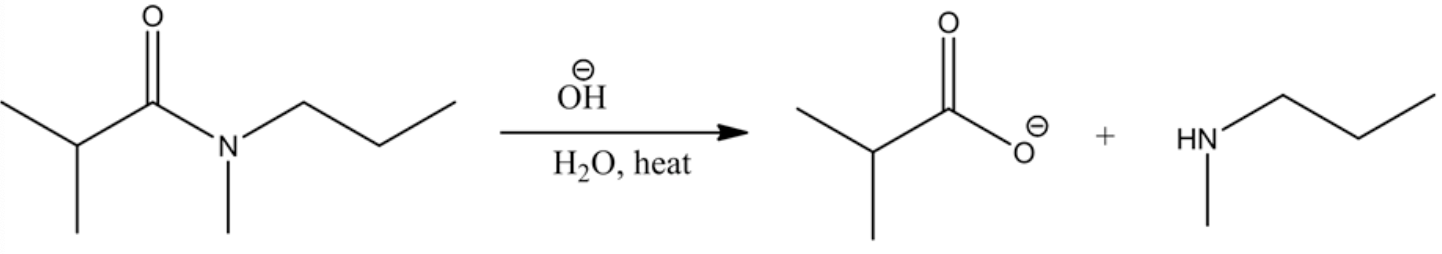
Stabilization of oxyanion to carboxylic acid is a slow step process while carboxylic acid to carboxylate conversion in the presence of a base is very rapid. An amide ion abstracts the acidic proton from the carboxylic acid very rapidly and forms a carboxylate ion. The carboxylate ion is highly stabilized through resonance.
The complete reaction
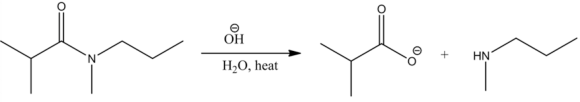
Related Solutions
For the following SN2 reaction, draw the organic and inorganic products of the reaction, and identify...
Draw the structures of the organic products in each reaction of the following two-step synthesis.
For the following SN2 reaction, draw the organic and inorganic products of the reaction, and identify the nucleophile, substrate, and leaving group.
For the following SN2 reaction, draw the organic and inorganic products of the reaction, and identify the nucleophile, substrate, and leaving group.
Draw the major organic product(s) for the following reaction. Multiple products may be drawn in one...
Draw the organic products formed when allylic alcohol is treated with each reagent a. H2 +...
Draw the major organic product of the reaction shown below.Draw the major organic product of...
Draw the organic product for the following reaction. Omit any inorganic byproducts or ions.
Draw the structure of the organic product of each reaction in the following two-step synthesis.
draw a mechanism that explains how the nitro-substituted aromatic products observed in your reaction were formed
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago