Question
In: Chemistry
Draw the structure of the aromatic product from the following reaction
Draw the structure of the aromatic product from the following reaction
Solutions
Expert Solution
Concepts and reason
An acid anhydride reacts with amines to form an amide bond. Amide bond consists of CONH group, in which \(\mathrm{NH}\) group acts as a nucleophile and \(\mathrm{CO}\) group acts as an electrophile.
Fundamentals
Acid anhydrides are the compounds that are produced by removing water molecules from the acid and these act as nucleophiles. When an acid anhydride reacts with an amine, it forms a peptide bond.
The given substrate is shown below.
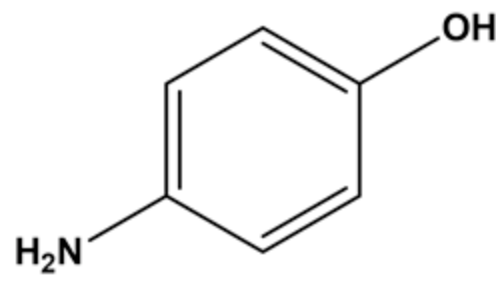
The given substrate consists of two functional groups namely \(\mathrm{OH}\) and \(\mathrm{NH}_{2}\). The amine group \(\left(\mathrm{NH}_{2}\right)\) is more nucleophilic than \(\mathrm{OH}\) since the electron density around \(\mathrm{NH}_{2}\) is more than \(\mathrm{OH}\).
The reaction can be written as follows.
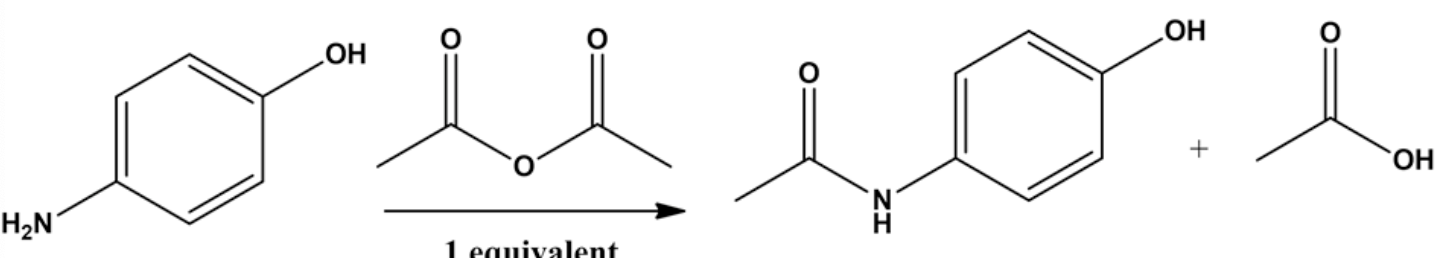
The structure of the aromatic product is

The amine group is more nucleophilic than \(\mathrm{OH}\) group. Hydrogen can be removed from amine \(\left(\mathrm{NH}_{2}\right)\) group thereby electrophilic attack of anhydride takes place resulting in the formation of the peptide bond.
The structure of the aromatic product is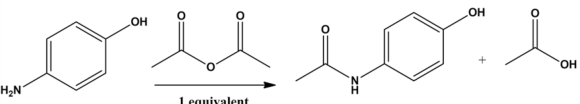
Related Solutions
Nitration of an aromatic ring involves an electrophilicsubstitution reaction. Draw the structure of the electrophile...
Draw the structure of the organic product of each reaction in the following two-step synthesis.
Propose a structure for an aromatic hydrocarbon, C9H12, that can form only one C9H11Br product on...
draw a mechanism that explains how the nitro-substituted aromatic products observed in your reaction were formed
Draw the reaction diagram from starting material to product of your specific reagent. This is for...
Draw the organic product for the following reaction. Omit any inorganic byproducts or ions.
What are the electrophile and nucleophilic in the electrophilic aromatic substitution reaction.
Draw the product of the oxidation of the following aldehyde. Include all hydrogen atoms in your structure.
Draw the major organic product of the reaction shown below.Draw the major organic product of...
Draw the organic product (if any) expected from the following reaction: (include all hydrogen atoms) CH3CH2CH2OH + K2Cr2O7(aq) H2SO4
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago