Question
In: Chemistry
N-Methyl-2-pyrrolidone is an aprotic solvent used in many industrial processes. Draw the structure of the product formed when it is heated with strong aqueous acid 6MHCI, H20 and heat.
N-Methyl-2-pyrrolidone is an aprotic solvent used in many industrial processes. Draw the structure of the product formed when it is heated with strong aqueous acid 6MHCI, H20 and heat.
Solutions
Expert Solution
Concepts and reason
This problem is based on the concept of the reaction of \(\mathrm{N}\) -methyl- 2 -pyrrolidone. N-methyl-2-pyrrolidone is an organic compound that consists of a five-membered lactam. It is called NMP as short. It is a beneficial compound that is used to recover hydrocarbons generated during the processing of petrochemicals.
Fundamentals
N-methyl-2-pyrrolidone is a very reactive compound. It reacts in an acidic medium to form a variety of products. Some reactions involve the ring-opening step.
The structure of N-methyl-2-pyrrolidone is given below:
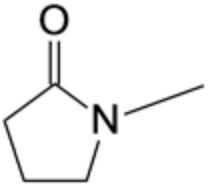
The compound has a five-membered ring, which consists of four carbon atoms and one nitrogen atom. The ring has one oxygen attached to it via the double bond, and one methyl group is attached to the nitrogen atom.
The chemical reaction involved is as follows:
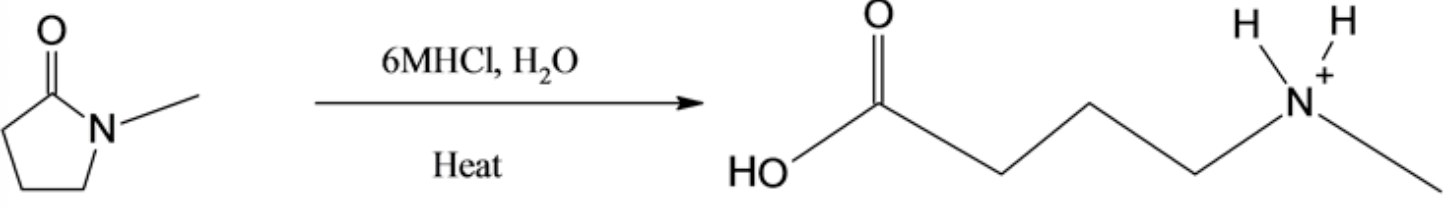
The structure of the product is given below:
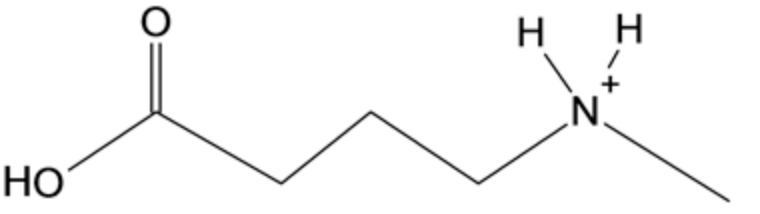
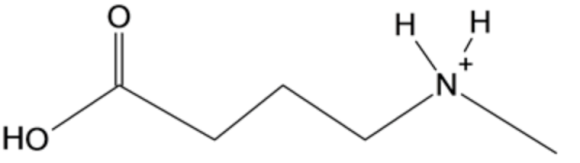
N-methyl-2-pyrrolidone on reaction with strong aqueous acidic medium followed by the treatment of heat results in the opening of the ring and forms a compound having a carboxylic group at one end and protonated nitrogen atom which has one methyl group attached to it, at another end.
The structure of the product is given below:
Related Solutions
Butanone, when treated with methyl magnesium bromide and thendilute aqueous acid, yields 2-methyl-2-butanol.a) Please...
draw full sn1 mechanism for a reaction that includes 2-methyl-2-propanol, n-pentanol and hydrochloric acid as the...
1) Draw the structure of the product formed by addition of vitamin B1 and benzaldehyde. 2)...
1) Draw the structure of the product formed by addition of vitamin B1 and benzaldehyde. 2)...
1)When optically active (R)-2- methyl cyclohexanone is treated with aqueous acid , its optical activity is...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago