Question
In: Other
What does it mean for example: 100% of methane and oxygen entering are consumed by reactions...
What does it mean for example: 100% of methane and oxygen entering are consumed by reactions 1 and 2..
Or another example... 95% of hydrogen entering is consumed...
Solutions
Expert Solution
Answer:
It simply means the all methane and oxygen entering the system is consumed by the reactions.
For example: (Assume reaction is very fast, don't get confused with time).
- If complete combustion of methane ( CH4 +
2O2
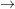 CO2 + 2H2O ) takes place in a reactor and if
25 mol/s of methane and 50 mol/s are fed to the system. From the
above combustion reaction 2 mol of oxygen react with 1 mol of
methane. Therefore, 50 mol of oxygen react with 25 mol of methane
i.e., all methane and oxygen entering the reactor is 100 % consumed
leaving nothing in the product stream.
CO2 + 2H2O ) takes place in a reactor and if
25 mol/s of methane and 50 mol/s are fed to the system. From the
above combustion reaction 2 mol of oxygen react with 1 mol of
methane. Therefore, 50 mol of oxygen react with 25 mol of methane
i.e., all methane and oxygen entering the reactor is 100 % consumed
leaving nothing in the product stream. - Now, consider the case when 30 mol/s of methane and 80 mol/s of oxygen is fed to the reactor and assume that complete combustion of methane takes place. From the stiochiometry of the above reaction, 1 mol of methane reacts with 2 moles of oxygen. Therefore, 30 mol of methane will react with 60 mol of oxygen. 20 mol of oxygen are left unreacted i.e., 100 % of methane entering the reactor and [{60/80} x100] 75 % of oxygen entering the reactor is consumed by the reaction.
If the reactant is comsumed fully (100%) the reactant can not be in the product stream. If it is consumed partially say 90 % then10 % of unreacted reactant will in the product stream.
It is not necessary that the reactants will be consumed fully if we fed reactants in the ratio same as of their stichiometric ratio. There may be several causes like if the rate of reaction is larger than the rate at which reactants are fed to the system, partial combustion, undisered reaction which may use reactants in different stichiometric ratios etc. I hope you may be aware of the terms like limiting reagent and excess reagent.
If you have still any douby feel free to ask me.
Related Solutions
During the soybean experiment the nongerminating soybeans consumed around 0.02 mL/min of oxygen. What does this...
What does it mean to declare a variable? Give an example.
what percentage of bound oxygen does hemoglobin release between 100 and 30 torr of pO2? a....
QUESTION 63 Carbon fixation occurs during the “dark reactions”. What does this mean? a. Sugar is...
1.When 2.18 g of methane burns in oxygen, 109 kJ of heat is produced. What is...
What does the term "Oppression Olympics" mean? and give example. What does the term "intersectionality" off...
What does perpetuity mean? Please describe a financial example of a perpetuity.
What does it mean to have a “future taxable amount” and give an example of a...
1.Write characteristics of Active transport with example. 2.What do you mean by exergonic and endergonic reactions?...
16. What does it mean to say that figures should be reconciled? (100 words)
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
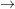 CO2 + 2H2O ) takes place in a reactor and if
25 mol/s of methane and 50 mol/s are fed to the system. From the
above combustion reaction 2 mol of oxygen react with 1 mol of
methane. Therefore, 50 mol of oxygen react with 25 mol of methane
i.e., all methane and oxygen entering the reactor is 100 % consumed
leaving nothing in the product stream.
CO2 + 2H2O ) takes place in a reactor and if
25 mol/s of methane and 50 mol/s are fed to the system. From the
above combustion reaction 2 mol of oxygen react with 1 mol of
methane. Therefore, 50 mol of oxygen react with 25 mol of methane
i.e., all methane and oxygen entering the reactor is 100 % consumed
leaving nothing in the product stream. Amanda K answered 3 years ago
Amanda K answered 3 years ago