Question
In: Chemistry
An ethylene glycol solution contains 21.2 g of ethylene glycol (C2H6O2) in 89.4 mL of water....
An ethylene glycol solution contains 21.2 g of ethylene glycol (C2H6O2) in 89.4 mL of water. (Assume a density of 1.00 g/mL for water.)
Determine the boiling and freezing point of the solution. Express you answer in degrees Celsius.
Solutions
Expert Solution
Number of moles of ethylene glycol (C2H6O2)=2xMolar mass of C+6xMolar mass of H+2xMolar mass of O
=2x12 g/mol+6x1 g/mol+2x16 g/mol
=24 g/mol+6 g/mol+32 g/mol
=62 g/mol
Number of moles of ethylene glycol=mass of ethylene glycol/molar mass of ethylene glycol
=21.2 g/62 g/mol=0.34 mol
Mass of water=Volume x density
=89.4 mL x 1.00 g/mL=89.4 g
Molality of given solution=number of moles of ethylene glycol/mass of water (kg)
=0.34 mol/(89.4 g/1000 g/kg) (1 kg=1000 g)
=3.8 m
Freezing point of pure water=0°C
Freezing point depression constant for water=Kf=1.86 °C/m
We know that freezing point depression is given as
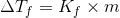
Where m=Molality of solution
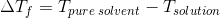 =Kf
x m
=Kf
x m
Here Tpure solvent=freezing point of pure water=0°C
Tsolution =Freezing point of solution
0°C-Tf=1.86°C/m x 3.80 m=7.1°C
Tf=0°C-7.1°C=-7.1°C
So freezing point of given solution=-7.1°C
Boiling point of pure water=100°C
Boiling point elevation constant for water=Kb=0.512 °C/m
Elevation in boiling point is given as
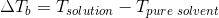 =Kb
x m
=Kb
x m
Where Tsolution=Boiling point of solution
Tpure solvent=Boiling point of pure water =100°C
m=Molality of solution
Tb-100°C=0.512°C/m x 3.8 m=1.9 °C
Tb=1.9°C+100°C=101.9°C
So boiling point of given solution=101.9°C
Related Solutions
An ethylene glycol solution contains 16.2 g of ethylene glycol (C2H6O2) in 87.4 mL of water....
An ethylene glycol solution contains 27.6 g of ethylene glycol (C2H6O2) in 90.4 mL of water....
An ethylene glycol solution contains 29.8 g of ethylene glycol (C2H6O2) in 92.6 mL of water....
An ethylene glycol solution contains 24.6 g of ethylene glycol (C2H6O2) in 89.8 mL of water....
A 1.5-Liter solution contains 45.0 mL of ethylene glycol. What is the volume percent of ethylene...
What is the mole fraction of ethylene glycol, C2H6O2
Ethylene glycol, formula C2H6O2, is used as antifreeze for automobiles and is sometimes mixed with water...
Ethylene glycol, formula C2H6O2, is used as antifreeze for automobiles and is sometimes mixed with water...
A water sample contains 150 mg/L ethylene glycol (C2H6O2) and 200 mg/L phenol (C6H6O). Calculate the...
How many grams of ethylene glycol (C2H6O2) must be added to 1.20 kg of water to...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
 queen_honey_blossom answered 3 months ago
queen_honey_blossom answered 3 months ago