Question
In: Chemistry
Compressibility, τ, is defined as: τ = − 1 /ν *(dν/ dp) Show that the ratio...
Compressibility, τ, is defined as: τ = − 1 /ν *(dν/ dp) Show that the ratio of the isothermal compressibility to the isentropic compressibility is equal to the specific heat ratio for a perfect gas.
Solutions
Expert Solution

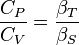
Here  is the thermal expansion coefficient:
is the thermal expansion coefficient:

 is the isothermal compressibility:
is the isothermal compressibility:

and  is the isentropic compressibility:
is the isentropic compressibility:
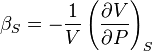
A corresponding expression for the difference in specific heat capacities (intensive properties) at constant volume and constant pressure is:

where ρ is the density of the substance under the applicable conditions.
The corresponding expression for the ratio of specific heat capacities remains the same since the thermodynamic system size-dependent quantities, whether on a per mass or per mole basis, cancel out in the ratio because specific heat capacities are intensive properties. Thus:

The difference relation allows one to obtain the heat capacity for solids at constant volume which is not readily measured in terms of quantities that are more easily measured. The ratio relation allows one to express the isentropic compressibility in terms of the heat capacity ratio.
Related Solutions
A. Show that the ideal gases following PV=nRT satisfy (dp/dv)(dv/dt)(dt/dp)=-1. B. Show that van see waals...
The compressibility of water is 4.4 x 10-10 Pa-1. The compressibility for clay, gravel...
Show that dg = -sdT + vdp is equivalent to dg = v(dp/dv)dv + (v(dp/dt)-s)dt gibbs...
Explain the trend in CO stretches below. ν(CO) = 2143 cm-1 in CO ν(CO) = 2090...
(The “conjugation rewrite lemma”.) Let σ and τ be permutations. (a) Show that if σ maps...
Show that any two permutations σ,τ ∈ Sn have the same cycle structure if and only...
Let τ ∈ Sn be the cycle (1, 2, . . . , k) ∈ Sn...
Beta is defined as: the ratio of the variance of market returns to the covariance of...
The bad debt ratio for a financial institution is defined to be the dollar value of...
4. Unemployment rate is defined as the ratio of which of these: a) labor force...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 queen_honey_blossom answered 2 years ago
queen_honey_blossom answered 2 years ago