Question
In: Chemistry
#1 Consider the iron sulfur rubredoxins having a single iron center in their natural biological environment....
#1
Consider the iron sulfur rubredoxins having a single iron center in their natural biological environment. Which of the following statements is incorrect.
| The iron atom is usually found in a nearly tetrahedral geometry surrounded by sulfurs. |
| Iron(III) rubredoxins act as reducing agents during electron transfer reactions. |
| The iron(III) rubredoxins exhibit a larger paramagnetism than their corresponding iron (II) rubredoxins. |
| Rubredoxins are only capable of gaining or loosing a single electron. |
| Iron(II) and iron(III) rubredoxins are found as high-spin iron complexes. |
#2
Which of the following formal oxidation states are present under normal biological conditions for the most oxidized form of a Fe4S4 ferredoxin center?
| Fe(III)2Fe(II)2 |
| Fe(III)3Fe(II)1 |
| Fe(III)1Fe(II)3 |
| Fe(III)4 |
| Fe(II)4 |
Solutions
Expert Solution
Rubredoxins perform one-electron transfer processes. The central iron atom changes between the +2 and +3 oxidation states. In both oxidation states, the metal remains high spin, which helps to minimize structural changes.
The structue of iron in rubredoxins is

So it is tetrahedral.
Since the iron is in high spin state it will have unpaired electrons and so will be paramagnetic.
So the only statement among those given which is not true is
| Iron(III) rubredoxins act as reducing agents during electron transfer reactions. |
Because if Iron(III) rubredoxins act as reducing agents, the other compound gets reduced and so Iron(III) rubredoxins should get oxidised Iron(III) rubredoxins is already having iron in 3+ state it can not go to 2+ state which means it will get reduced so the other species should get oxidised so it acts as an oxidising agent.
2)
The [Fe4S4] ferredoxins may be further subdivided into low-potential (bacterial-type) and high-potential (HiPIP) ferredoxins.
Low- and high-potential ferredoxins are related by the following redox scheme:
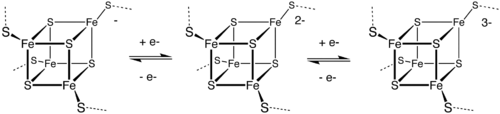
The formal oxidation numbers of the iron ions can be [2Fe3+, 2Fe2+] or [1Fe3+, 3Fe2+] in low-potential ferredoxins. The oxidation numbers of the iron ions in high-potential ferredoxins can be [3Fe3+, 1Fe2+] or [2Fe3+, 2Fe2+].
So the correct answer is
Fe(III)3Fe(II)1
Related Solutions
Suppose two blocks of iron, each having 1 mol of iron atoms, are at differing temperatures...
Manufacturing Technology 1. The effects of cutting fluids on biological considerations and the environment. 2. Find...
1. Although we teach natural selection using a single allele, we recognize that natural selection works...
1 Which of the following is an advantage of having a single set of accounting standards...
Consider a single murine hepatocyte at homeostasis with its surrounding environment, which includes other neighboring hepatocytes...
1. Consider a project having the following seven activities: &
1) Why are quantum dots generally created for biological use having a core-shell design? 2) Design...
1. How has the economic activity as a geographical force changed the natural environment? Provide example(s)...
1. (2 marks) (Covering part 1 of the lecture slides) Consider the subset A of natural...
Apple Inc.'s External Environment: what are Apple's (1) Natural, (2) Societal, and (3) Task Environments? (A...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 queen_honey_blossom answered 1 year ago
queen_honey_blossom answered 1 year ago