Question
In: Chemistry
Suppose two blocks of iron, each having 1 mol of iron atoms, are at differing temperatures...
Suppose two blocks of iron, each having 1 mol of iron atoms, are at differing temperatures and are then brought together in an otherwise isolated system. One of the blocks is at 273.15 K and the other is at 373.15 K and the process is carried out at 1 bar pressure.
a.) Would you expect ∆Ssys < 0, ∆Ssys = 0, ∆Ssys > 0 ?
b.) Using common sense and high school physics (and perhaps the First Law), what is the common final temperature of the two blocks?
c.) Calculate ∆Ssys for the process (use Table 4.1 for any needed molar heat capacities needed, and assume that the molar heat capacities are independent of T).
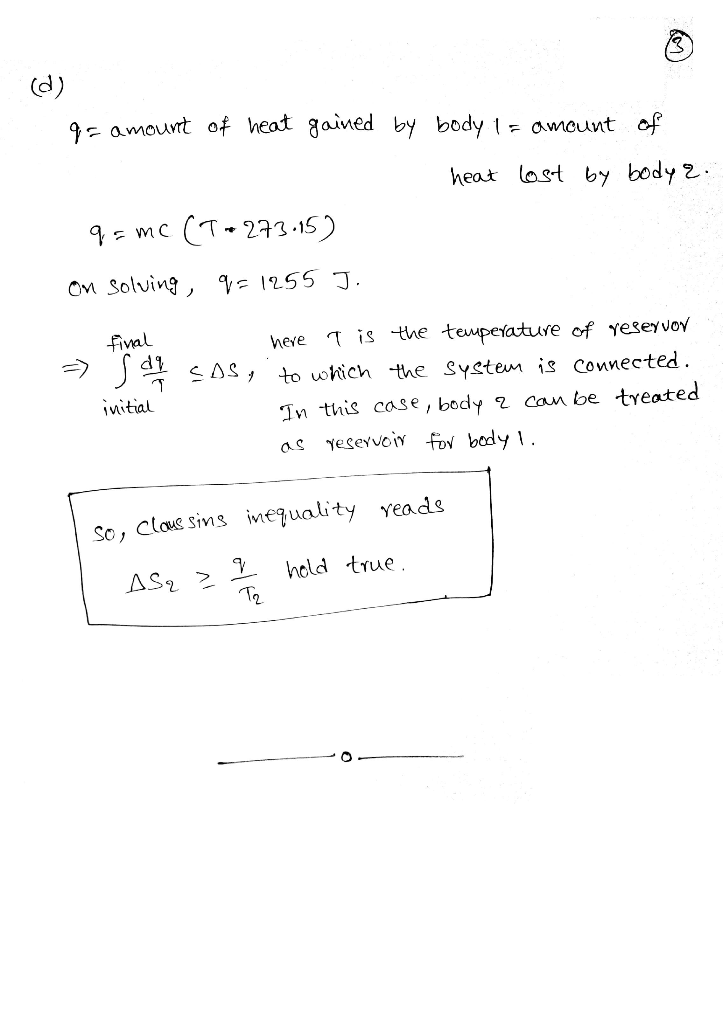
d.) What is q for the isolated system? How does this value of q and the result of part C relate to the Clausius inequality: final initial d q S T − ∆ ≥ ∫ ?
e.) Interpret this change in entropy in terms of the ordering-disordering effects involved in the transfer of given amount of heat from a block at higher temperature to one at a lower temperature (i.e. the disordering effect of adding a given q to the cooler block vs the ordering effect of removing the same q from the hotter block).
Molar heat capacity of Fe(s) ==> Cp,m (J / mol*K) = 25.1
Solutions
Related Solutions
Two copper blocks, each of mass 1.96 kg, initially have different temperatures,t1 = 17° C and...
The two ends of an iron rod are maintained at different temperatures. The amount of heat...
Suppose that some of the iron atoms in the ceramic FeO are oxidized from Fe 2+...
Suppose that some of the iron atoms in the ceramic FeO are oxidized from Fe 2+...
In FCC iron, carbon atoms are located at octahedral sites at the centre of each edge...
Two blocks are connected by a light string that passes over a frictionless pulley having a...
26/04/19 If two metal blocks of initially different temperatures come together and equilibrate to the same...
#1 Consider the iron sulfur rubredoxins having a single iron center in their natural biological environment....
Suppose a person’s life is divided into two main blocks, periods 1 and 2. The consumer...
Suppose a person’s life is divided into two main blocks, periods 1 and 2. The consumer...
- In long paragraphs answer the questions below: Discuss the key components (where, when, what) and causes...
- Sinkal Co. was formed on January 1, 2018 as a wholly owned foreign subsidiary of a...
- Larry’s best friend, Garfield, owns a lasagna factory. Garfield’s financial skills are not very strong, so...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...


 queen_honey_blossom answered 4 months ago
queen_honey_blossom answered 4 months ago