Question
In: Chemistry
Below are shown three Lineweaver-Burk plots for enzyme reactions that have been carried out in the presence, or absence, of an inhibitor.
Below are shown three Lineweaver-Burk plots for enzyme reactions that have been carried out in the presence, or absence, of an inhibitor.
Part A
Indicate what type of inhibition is predicted based on Lineweaver-Burk plot.
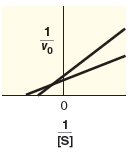
| mixed inhibition | |
| competitive inhibition | |
| uncompetitive inhibition |
Part B
Indicate which line corresponds to the reaction without inhibitor and which line corresponds to the reaction with inhibitor present.
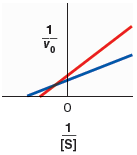
| red - without inhibitor, blue - with inhibitor | |
| blue - without inhibitor, red - with inhibitor |
Part C
Indicate what type of inhibition is predicted based on Lineweaver-Burk plot.
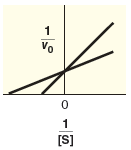
| mixed inhibition | |
| competitive inhibition | |
| uncompetitive inhibition |
Part D
Indicate which line corresponds to the reaction without inhibitor and which line corresponds to the reaction with inhibitor present.
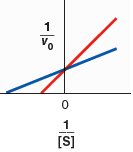
Indicate which line corresponds to the reaction without inhibitor and which line corresponds to the reaction
| blue - without inhibitor, red - with inhibitor | |
| red - without inhibitor, blue - with inhibitor |
Part E
Indicate what type of inhibition is predicted based on Lineweaver-Burk plot.
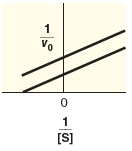
| mixed inhibition | |
| competitive inhibition | |
| uncompetitive inhibition |
Part F
Indicate which line corresponds to the reaction without inhibitor and which line corresponds to the reaction with inhibitor present.
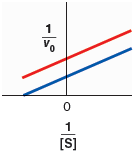
| blue - without inhibitor, red - with inhibitor | |
| red - without inhibitor, blue - with inhibitor |
Solutions
Expert Solution
Part-A
The lineweaver-Burk equation for the mixed inhibition is, \(\frac{1}{v_{0}}=\left(\frac{\alpha K_{m}}{V_{\max }}\right) \frac{1}{[s]}+\frac{\alpha^{\prime}}{V_{\max }}\)
Here, \(v_{0}\) is the initial velocity, \([s]\) is the substrate concentration,
$$ \alpha^{\prime}=\left(1+\frac{[\mathrm{I}]}{K_{I}^{\prime}}\right), \alpha=\left(1+\frac{[\mathrm{I}]}{K_{I}}\right) $$
\([\mathrm{I}]\) is the inhibitor concnetration, \(V_{\max }\) is the maximum velocity \(K_{I}^{\prime}\) and \(K_{I}^{\prime}\) are the dissociation constants, and \(K_{m}\) is the Michael's constant.
The corresponding lineweaver-Burk plot for the mixed inhibition is shown below:
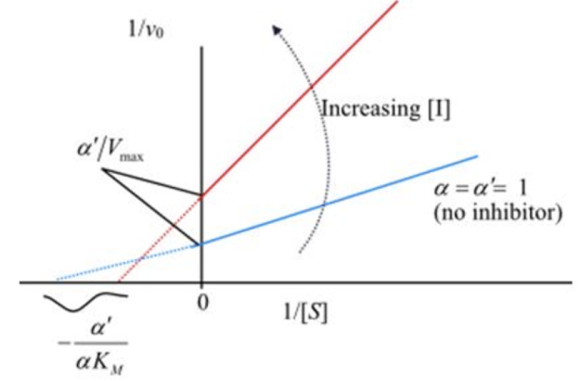
Plot 1: Lineweaver-Burk plot in the presence of mixed inhibitor
The curled arrow which is in anti-clock wise direction indicates increasing inhibitor concentration.
Compare the specified plot with the plot-1. Thus, based on the plot the enzyme reaction is a mixed inhibition type.
Part-B
Compare the specified plot with plot-1, Thus, the blue line indicates the line equation without inhibitor and, the red line indicates the line equation with inhibitor.
Part-C
The lineweaver-Burk equation for the competitive inhibition is, \(\frac{1}{v_{0}}=\left(\frac{\alpha K_{m}}{V_{\max }}\right) \frac{1}{[s]}+\frac{1}{V_{\max }}\)
The corresponding lineweaver-Burk plot for the competitive inhibition is shown below:

Plot 2: Lineweaver-Burk plot in the presence of a competitive inhibitor
Compare the specified plot with the plot-2. Thus, based on the plot the enzyme reaction is competitive inhibition type.
Part-D
Compare the specified plot with plot-2, Thus, the blue line indicates the line equation without inhibitor and, the red line indicates the line equation with inhibitor.
Part-E
The lineweaver-Burk equation for the uncompetitive inhibition is, \(\frac{1}{v_{0}}=\left(\frac{K_{m}}{V_{\max }}\right) \frac{1}{[s]}+\frac{\alpha^{\prime}}{V_{\max }}\)
The corresponding lineweaver-Burk plot for the uncompetitive inhibition is shown below:
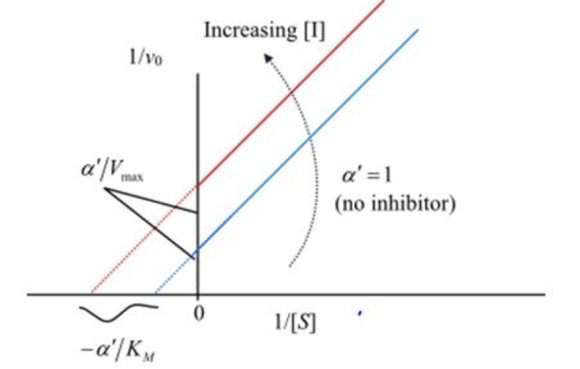
Plot 3: Lineweaver-Burk plot in the presence of the uncompetitive inhibitor
Compare the specified plot with the plot-3. Thus, based on the plot the enzyme reaction is an uncompetitive inhibition type.
Part-F
Compare the specified plot with plot-3, Thus, the blue line indicates the line equation without inhibitor and, the red line indicates the line equation with inhibitor.
Related Solutions
3. The kinetics of an enzyme are studied in the absence and presence of an inhibitor...
Students collected Beta-fructosidase enzyme activity data in the presence and absence of an unknown inhibitor. P-nitrophenol...
Verdigris, copper (II) acetate monohydrate, was prepared using the three reactions shown below. In a 125...
1) Three new-product ideas have been suggested. These ideas have been rated as shown in the...
The data shown below for the dependent variable, y, and the independent variable, x, have been...
The data shown below for the dependent variable, y, and the independent variable, x, have been...
You have been given the expected return data shown in the first table on the three...
he data shown below for the dependent variable, y, and the independent variable, x, have been...
Which are the three yield curve factors that have been shown to affect Treasury security returns...
Portfolio analysis You have been given the return data shown in the first table on three...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
- For this assignment you will write a program with multiple functions that will generate and save...
- How many grays is this?Part A A dose of 4.7 Sv of γ rays in a...
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago