Question
In: Chemistry
How is the shape of a monoprotic acid titration curve determined by the pk of the...
How is the shape of a monoprotic acid titration curve determined by the pk of the acidic proton?
Solutions
Expert Solution
Titration curves for strong acid vs. strong base
We'll take hydrochloric acid and sodium hydroxide as typical of a strong acid and a strong base.

Running acid into the alkali

You can see that the pH only falls a very small amount until quite near the equivalence point. Then there is a really steep plunge. If you calculate the values, the pH falls all the way from 11.3 when you have added 24.9 cm3 to 2.7 when you have added 25.1 cm
Running alkali into the acid
This is very similar to the previous curve except, of course, that the pH starts off low and increases as you add more sodium hydroxide solution.

Again, the pH doesn't change very much until you get close to the equivalence point. Then it surges upwards very steeply.
he equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant (usually a base). It can be calculated precisely by finding the second derivative of the titration curve and computing the points of inflection (where the graph changes concavity); however, in most cases, simple visual inspection of the curve will suffice (in the curve given to the right, both equivalence points are visible, after roughly 15 and 30 mL of NaOH solution has been titrated into the oxalic acid solution. To calculate the acid dissociation constant (pKa), one must find the volume at the half-equivalence point, that is where half the amount of titrant has been added to form the next compound (here, sodium hydrogen oxalate, then disodium oxalate). Halfway between each equivalence point, at 7.5 mL and 22.5 mL, the pH observed was about 1.5 and 4, giving the pKa.
In monoprotic acids, the point halfway between the beginning of the curve (before any titrant has been added) and the equivalence point is significant: at that point, the concentrations of the two species (the acid and conjugate base) are equal. Therefore, the Henderson-Hasselbalch equation can be solved in this manner:


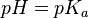
Related Solutions
how does titration of a strong monoprotic acid with a strong base differ from a titration...
1. How does the titration of a diprotic acid differ from a monoprotic acid (like HF)?...
While running the titration of your unknown monoprotic acid with NaOH, the pKa of the acid...
3. Plot a schematic titration curve for acetic acid with NaOH. Superimpose the titration curve of...
1.) Acetic acid is a weak monoprotic acid with Ka=1.8*10^-5. In an acid base titration, 100...
how many equivalence point(s) will appear on the titration curve during titration of phosphoric acid, H3PO4...
Titration without the PH meter: Calculate the molarity of your unknown, assuming it is monoprotic acid...
Oxalic acid is a common dibasic acid. Draw a qualitative sketch of the titration curve for...
REPORT FORM ; ACID TITRATION CURVE. Name of acid (or acid salt) NH4Cl mass of solid...
Draw a titration curve for both a strong acid titrated by a strong base and a...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
 queen_honey_blossom answered 2 years ago
queen_honey_blossom answered 2 years ago