Question
In: Chemistry
3. Plot a schematic titration curve for acetic acid with NaOH. Superimpose the titration curve of...
3. Plot a schematic titration curve for acetic acid with NaOH. Superimpose the titration curve of HCl on this graph. What are the main differences? How can we determine the pKa of acetic acid from its titration curve?
Solutions
Expert Solution
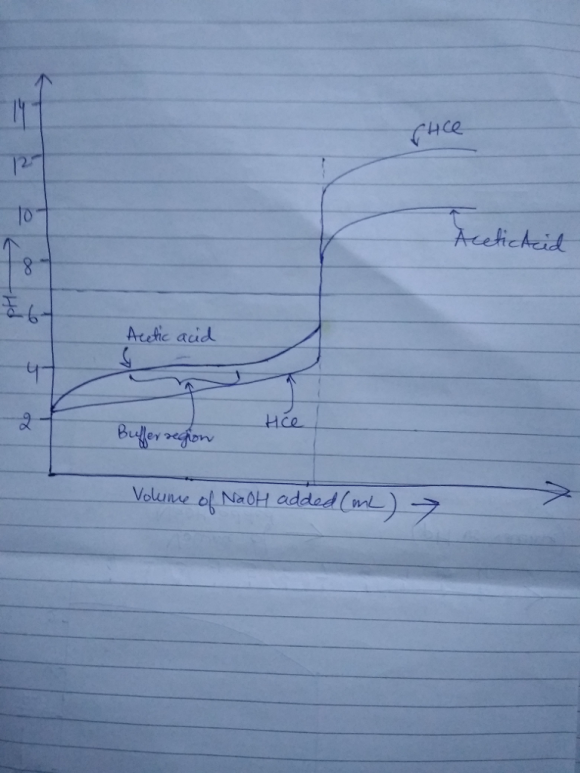
As we can see from the schematic diagram, for acetic acid, the change in pH is gradual near the buffer region, This is because in this buffer region, it resists the change in pH when NaOH is added.
But for HCl, there is no such buffer region and keeps on increasing until it reaches the equivalence point. At equivalence point, there is a sudden increase in pH.
After the equivalence point, when more of NaOH is added, then we see that pH increase is more for HCl.
This is becase in case of acetic acid, the following backward reaction also occurs:
CH3COO- + H2O ---> CH3COOH + OH-
So this causes the pH to be slightly less.
Related Solutions
sketch the general appearance of the curve for the titration of weak diprotic acid with NaOH....
For the titration of 75 mL of 0.10 M acetic acid with 0.10 M NaOH, calculate...
Exercise 1: Determining the Concentration of Acetic Acid Data Table 1. NaOH Titration Volume Initial NaOH...
Why does the curve of titration of acetic acid begin at a higher pH than the...
Consider the titration of 10.00 mL of 0.10 M acetic acid (CH3COOH) with 0.10 M NaOH...
Consider the titration of 25.0 mL of 0.100 M acetic acid (HA) with 0.100 M NaOH....
Titration Curve of unknown Acid Molarity of NaOH =.172 Trial 1 Trial 2 Mass of acid...
3. What would the titration curve look like if 0.10 M NaOH were in the flask...
Question 3: Draw the titration curve (pH versus mL of NaOH added) that would be obtained...
While running the titration of your unknown monoprotic acid with NaOH, the pKa of the acid...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago