Question
In: Chemistry
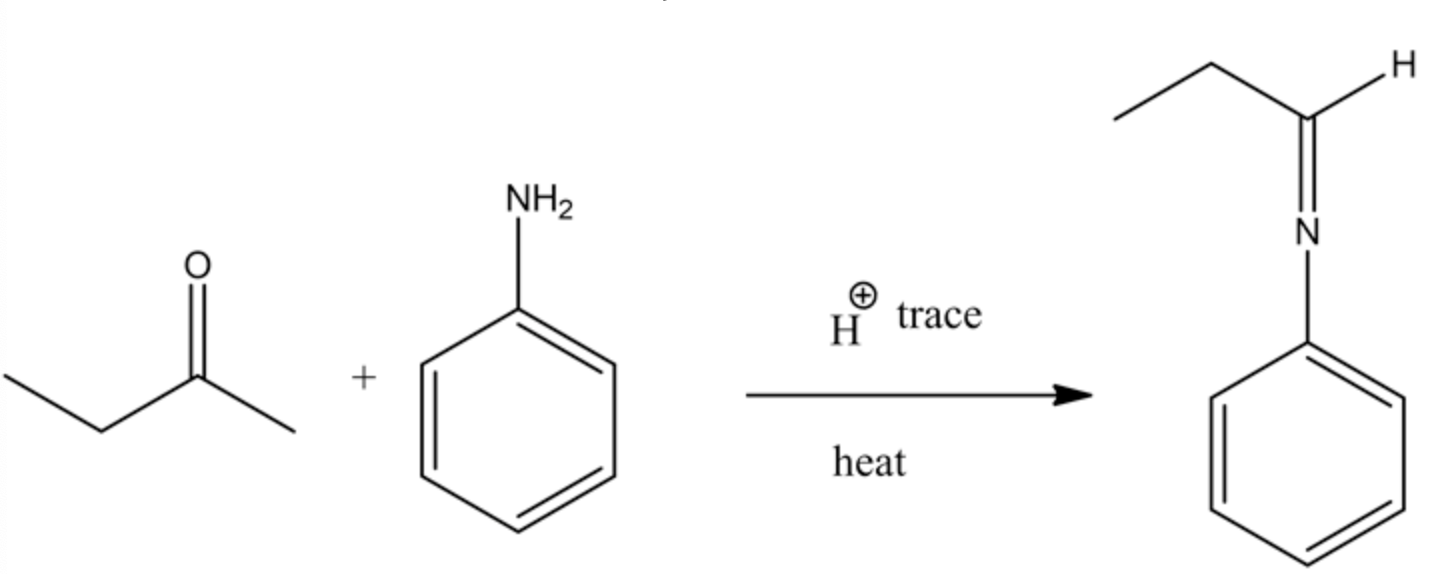
Draw the structures of the organic products in each reaction of the following two-step synthesis.
Draw the structures of the organic products in each reaction of the following two-step synthesis. (Hint: The nucleophilic amine attacks the electrophilic carbonyl carbon, and the resulting intermediate undergoes a proton shift and dehydration. Recall what is eliminated in a dehydration process. The second step is a reduction reaction; the reducing agent is NaBH3CN (or H2, metal catalyst). The aromatic ring is unaffected by these reagents. Look at the product of the first reaction and consider what can be easily reduced.)
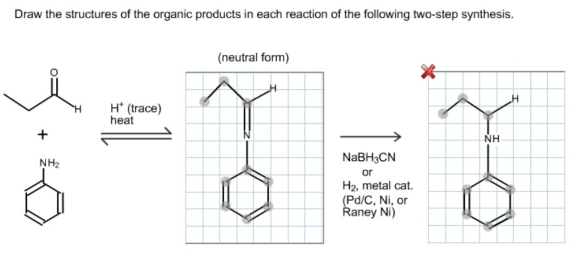
Solutions
Expert Solution
Concepts and reason
Major types of organic reactions are addition, substitution, elimination, condensation, rearrangement, oxidation and reduction reactions.
A condensation reaction is a type of organic reaction where an electron-deficient carbon atom reacts with an electron-rich reactant (nucleophile) to form a large molecule and remove a small molecule. In organic chemistry, a reduction reaction is a type of chemical reaction where a carbon atom gains bonds with less electronegative elements (hydrogen).
Fundamentals
The condensation reaction of carbonyl compounds and amines is where an electron-deficient carbon atom easily reacts with an electron-rich nucleophile to form a double bond. The carbon atom carries a partial positive charge and the oxygen atom carries a partial negative charge. These electronegative properties make the carbon atom an electrophilic center. The general reaction mechanism is given below.
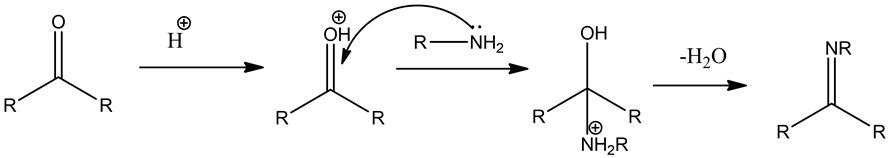
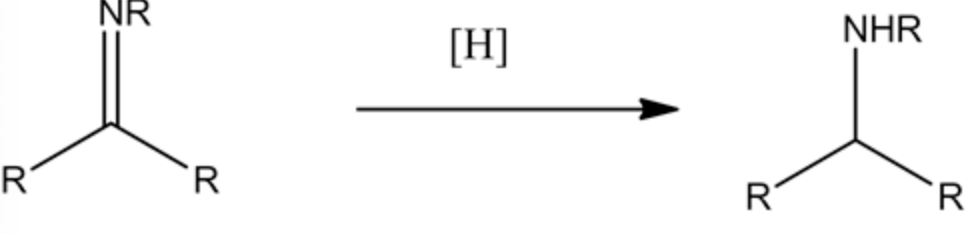
A nucleophile attacks the electron-deficient carbonyl carbon and is followed by dehydration to form a Schiff base form product. The carbon-nitrogen double bond undergoes a reduction reaction in the presence of a reducing agent. The reaction is as follows:
Acid catalyst protonates the oxygen atom in the aldehyde then produces an electron-deficient carbon atom. The lone pair of amine (base) nucleophiles attack on electron-deficient carbon atom followed by dehydration to form a Schiff base.
The reduction of the Schiff base is given below:
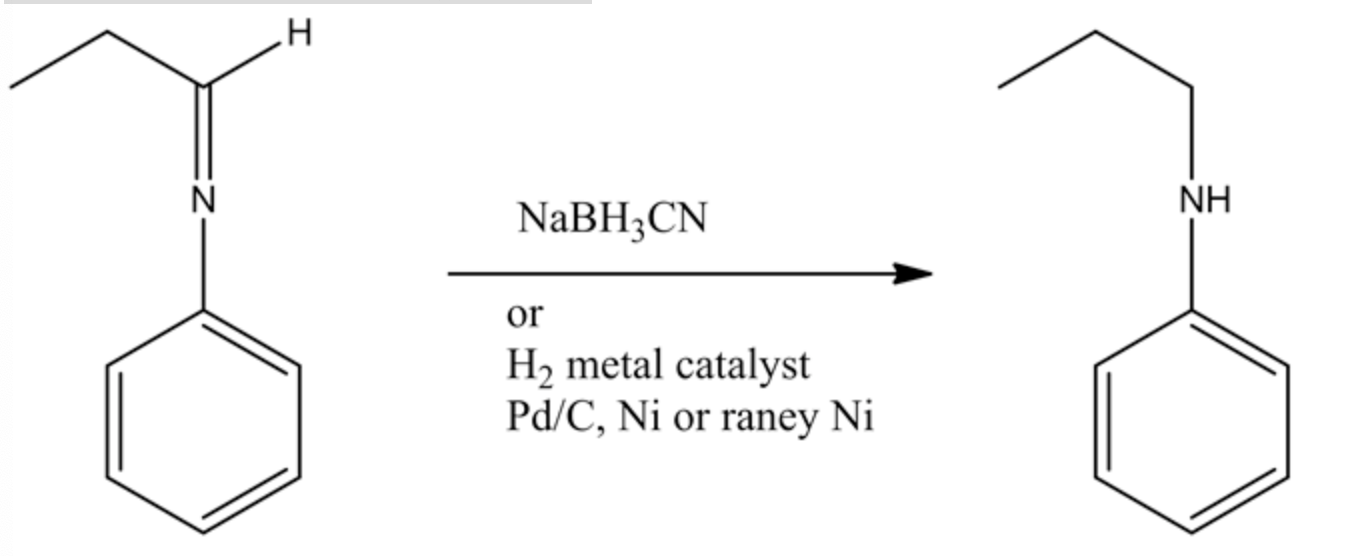
The Schiff base is reduced by using sodium cyanoborohydride and forming a secondary amine product (n-propyl aniline). This reducing agent precisely reduces the C=N double bond and does not reduce the benzene ring double bonds. So the final product formed in this reaction is n-propylaniline.
The structure of the organic product of the given reaction is
Related Solutions
Draw the structure of the organic product of each reaction in the following two-step synthesis.
Draw the organic products formed in the following reaction.
For the following SN2 reaction, draw the organic and inorganic products of the reaction, and identify...
BIOCHEMISTRY Draw structures and write the overall reaction in the citric acid cycle for each step...
For the following SN2 reaction, draw the organic and inorganic products of the reaction, and identify the nucleophile, substrate, and leaving group.
For the following SN2 reaction, draw the organic and inorganic products of the reaction, and identify the nucleophile, substrate, and leaving group.
ORGANIC CHEMISTRY: Draw a balanced chemical reaction for the synthesis of methyl salicylate from aspirin tablets....
Draw the major organic product(s) for the following reaction. Multiple products may be drawn in one...
Draw the two major allylic alcohol intermediates and the two final products in the following two-step...
For the following SN1 reaction, draw the major organic product, identify the nucleophile, substrate, and leaving group, and determine the rate limiting step.
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago