Question
In: Chemistry
Primary amines can be converted into secondary amines by reaction with haloalkanes. This reaction is difficult to achieve in the lab because of the large number of byproducts.
Primary amines can be converted into secondary amines by reaction with haloalkanes. This reaction is difficult to achieve in the lab because of the large number of byproducts. Select the possible products/ byproducts of the following reaction.
Solutions
Expert Solution
Concepts and reason
The concept used to solve this problem is the knowledge of nucleophilic substitution reaction. The \(\mathrm{S}_{\mathrm{N}} 2\) reaction or the bimolecular nucleophilic substitution reaction is a concerted type reaction followed by the trigonal bipyramidal shaped transition state which results in the formation of the product of inverted stereochemistry.
Fundamentals
Nucleophilic substitution reaction involves the primary attack of a nucleophile to the \(\sigma^{*}\) antibonding orbital of the carbon of alkyl halide, which on effect attach the nucleophile on that carbon and the removal of the halogen as a leaving group.
Attack of lone pair of nitrogen of substituted ammonia to the carbon- halogen bond of the halogen alkane. The initial attack is shown below: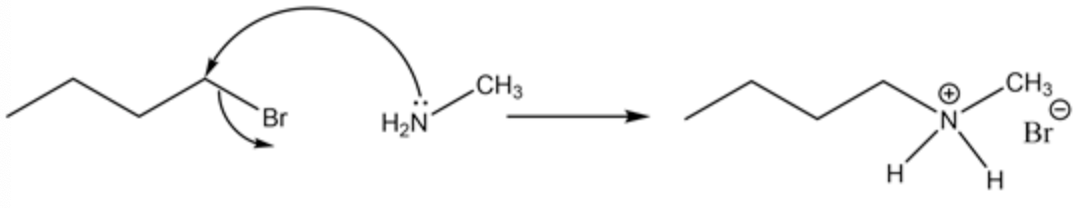
The lone pair of substituted ammonia initially attack the \(\sigma^{*}\) antibonding orbital of the carbon of alkyl halide which results in the removal of the halide group from the alkane chain while the nitrogen group gets attached to the ring results in the formation of salt alkyl ammonium halide.
Attack of the substituted ammonia acts as a base and abstracts proton from the alkyl ammonium halide salt to form the secondary amine. The attack of substituted ammonia is shown below:
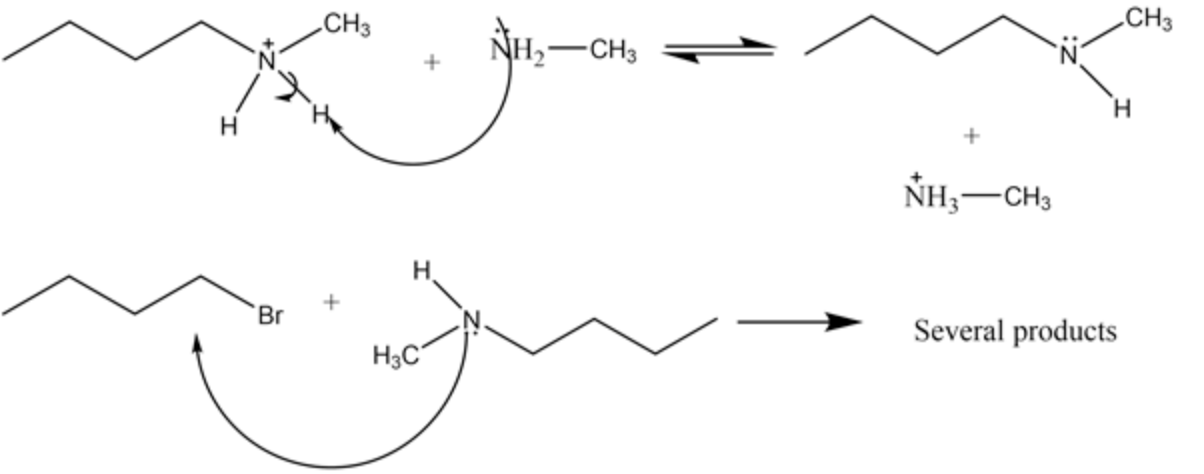
As each of the intermediates are stable and the product again acts as a nucleophile and hence several byproducts are possible.
The possible products and the byproducts formed are A, B, C, F, H, and K.
The alkyl ammonium salt is further attacked by the substituted ammonia which acts as a base and abstracts proton to form primary amine or secondary amine. This product can again act as a nucleophile and attack again to the alkyl halide to give several products.
The possible products and the byproducts formed are A, B, C, F, H, and K.
Related Solutions
Why is a secondary amine more nucleophilic than primary amines?
Nitroprusside will not work with primary and secondary amines if the pH is acidic. Why? (chemical...
Classify the lab tests listed by the phase of hemostasis it assesses (primary hemostasis, secondary hemostasis,...
1. Consider that a molecule of glutamate can be converted to alpha ketoglutarate by the reaction...
Private businesses have a great interest in quality primary and secondary education because today’s students are...
describe how the sense of control (primary vs secondary) can change with aging
1- Bromobutane sintesis Lab VII. Write the equations (chemical equations) taking place on the SECONDARY REACTION...
Assume that at the start of the reaction ΔG is a large negative number. As the...
Coke can be converted into CO�a fuel gas�in the reaction CO2 (g) + C (s) ?...
Exercises 1. A 25 kVA single-phase transformer has the primary and secondary number of turns of...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago