Question
In: Chemistry
Complete the following reaction. Add hydrogen atoms and charges to the appropriate atoms.
Complete the following reaction. Add hydrogen atoms and charges to the appropriate atoms.
Solutions
Expert Solution
Concepts and reason
The problem is based on the concept of calculation of the hydrolysis of the amide group. Hydrolysis of amide group takes place differently in acidic and basic medium.
Fundamentals
Hydrolysis of the amide group in acidic medium results in the formation of carboxylic acid and ammonium salt. Hydrolysis of the amide group in basic medium results in the formation of carboxylate ion and an amine.
Part a
The following reaction takes place when N-methylpropanamide reacts with water in a basic medium (presence of NaOH)
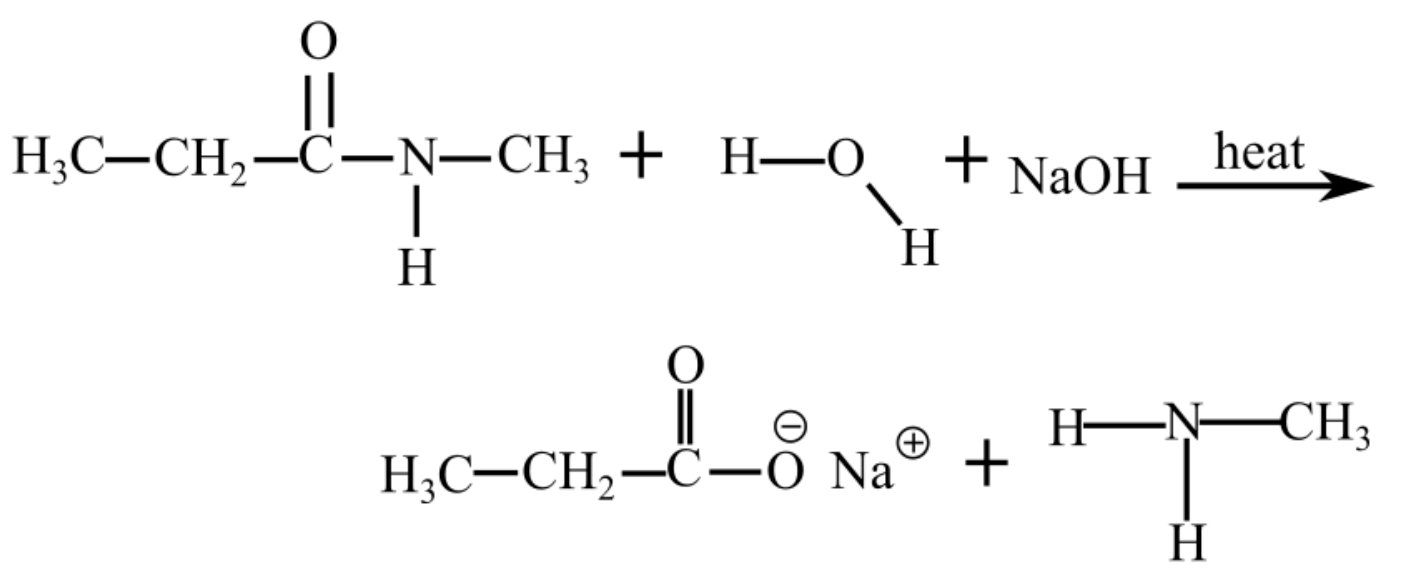
The products of hydrolysis of N-methylpropanamide in the basic medium are shown as follows:
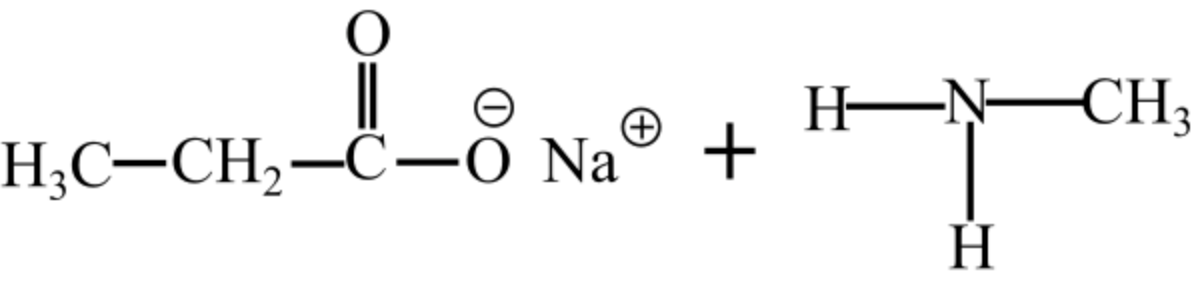
Hydrolysis of an alkanamide leads to the formation of a carboxylic acidic and an amine. In the basic medium, the carboxylic acid is converted to carboxylate ion and in the acidic medium, the amine group is converted to ammonium ion.
Part b
The following reaction takes place when N-methylpropanamide reacts with water in an acidic medium (presence of HCl)
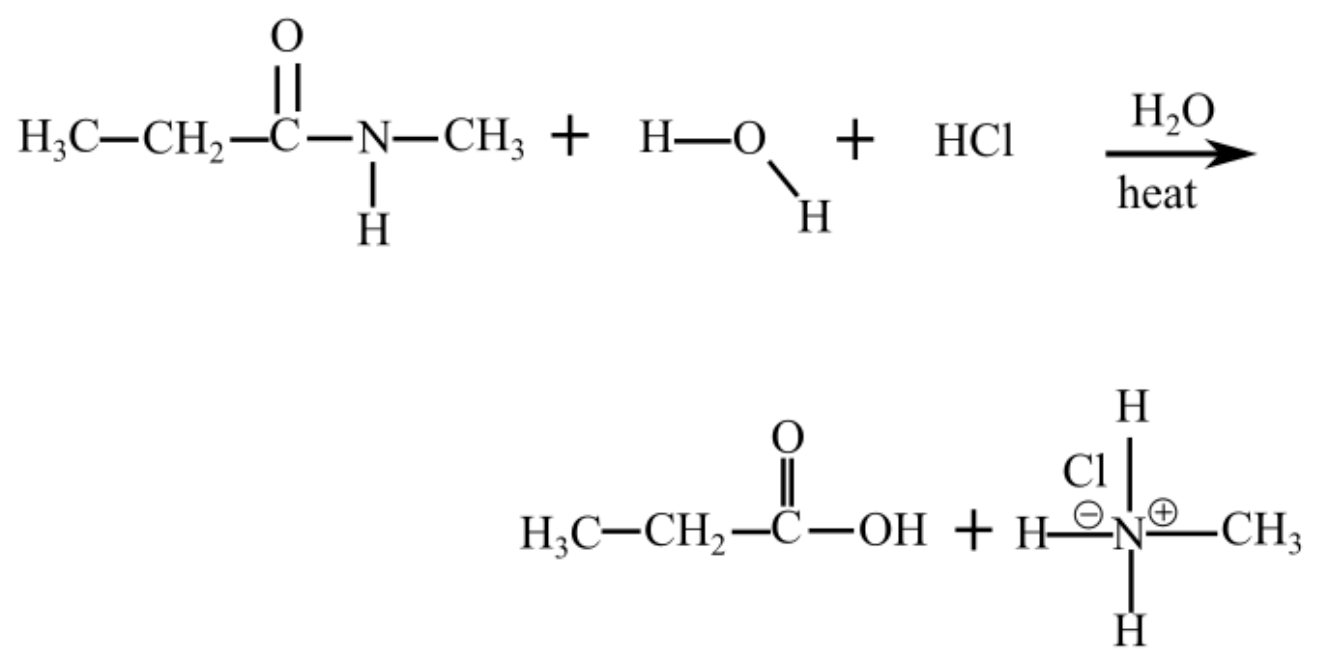
The products of hydrolysis of N-methylpropanamide are in acidic medium shown follows:
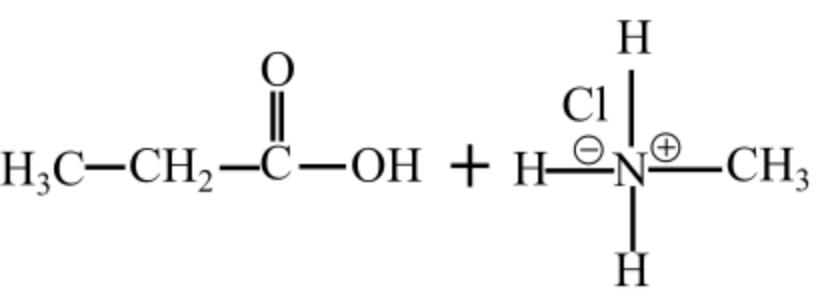
Hydrolysis of an alkanamide leads to the formation of a carboxylic acidic and an amine. In the basic medium, the carboxylic acid is converted to carboxylate ion and in the acidic medium, the amine group is converted to ammonium ion.
Part a
The products of hydrolysis of N-methylpropanamide in basic medium are shown follows:
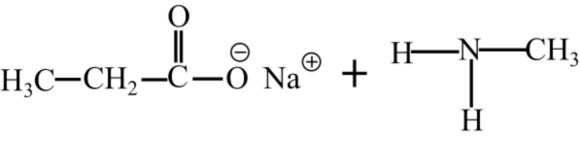
Part b
The products of hydrolysis of N-methylpropanamide are in acidic medium shown follows:
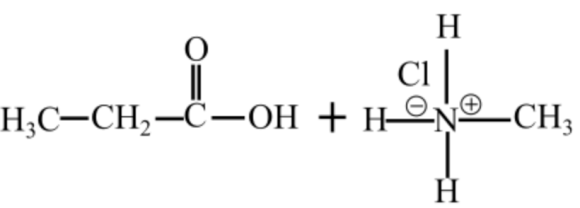
Related Solutions
Add electron dots and charges as necessary to show the reaction of calcium and oxygen to...
Add electron dots and charges as necessary to show the reaction of calcium and oxygen to...
Fill in the blanks 1. the ____ atoms of the ____ groups and the hydrogen atoms...
The sun is mostly composed of hydrogen-1 atoms. When two of these hydrogen-1 atoms collide, it...
Draw the organic product (if any) expected from the following reaction: (include all hydrogen atoms) CH3CH2CH2OH + K2Cr2O7(aq) H2SO4
Describe the acidity and pKa of hydrogen atoms that are bonded to carbon atoms adjacent to...
Add electron dots and charges as necessary to show the reaction of potassium and bromine to form an ionic compound.
Draw several ammonia molecules in Lewis structure, then add in some hydrogen bonds where appropriate; label...
Propanol- C3H7OH- Determine the ratio of atoms of Carbon per total atoms, Hydrogen per total atoms...
Which spectral lines in the emission spectrum of hydrogen atoms can be observed if the atoms...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago