Question
In: Physics
Find p1 the gauge pressure at the bottom of tube 1
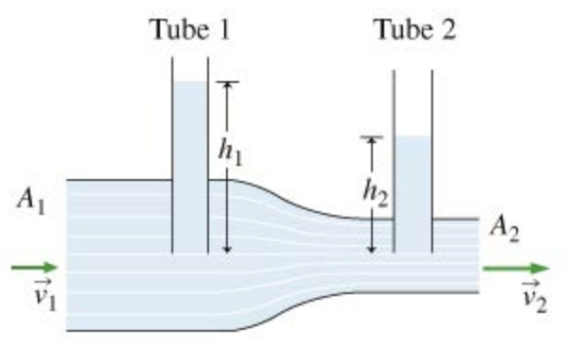
A pair of vertical, open-ended glass tubes inserted into a horizontal pipe are often used together to measure flow velocity in the pipe, a configuration called a Venturi meter. Consider such an arrangement with a horizontal pipe carrying fluid of density \(p\). The fluid rises to heights \({h_{1}}\) and \({h_{2}}\) in the two open-ended tubes (see figure). The cross-sectional area of the pipe is A1 at the position of tube 1, and \({A_{2}}\) at the position of tube 2.
Part A.Find \({p_{1}}\), the gauge pressure at the bottom of tube 1. (Gauge pressure is the pressure in excess of outside atmospheric pressure.)
Express your answer in terms of quantities given in the problem introduction and \({g}\), the magnitude of the acceleration due to gravity.
Part B.Find \({v_{1}}\), the speed of the fluid in the left end of the main pipe.
Express your answer in terms of \({h_{1}},{h_{2}}, g,\) and either \({A_{1}}\) and \({A_{2}}\) or \(\gamma\), which is equal to \(\frac{A_{1}}{A_{2}}\)
Solutions
Expert Solution
Given data
the density of the liquid is \(\rho\)
the height of the liquid in tube 1 is \(h\)
the height of the liquid in tube 2 is \(h_{2}\)
the cross-sectional area of the pipe on the left side \(A_{1}\)
the cross-sectional area of the pipe on the right side \(A_{2}\)
(a) The pressure at the bottom of the tube is
$$ p_{1}=p_{0}+\rho g h_{1} $$
Therefore the guage pressure is \({\rho g h_{1}}\)
(b) By using bernoulli's equation
$$ \mathrm{p}_{1}+\frac{1}{2} \rho v_{1}^{2}+\rho g y_{1}=\mathrm{p}_{2}+\frac{1}{2} \rho v_{2}^{2}+\rho g y_{2} $$
The potential terms are di sappear since the tube is horizontal
$$ \mathrm{p}_{1}+\frac{1}{2} \rho v_{1}^{2}=\mathrm{p}_{2}+\frac{1}{2} \rho v_{2}^{2} $$
The pressure at the tube 2 bottom is
$$ \mathrm{p}_{2}=p_{1}-\rho g\left(h_{1}-h_{2}\right) $$
From continuity equation
$$ \begin{aligned} A_{1} v_{1} &=A_{2} v_{2} \\ v_{2} &=\left(\frac{A_{1}}{A_{2}}\right) v_{1} \end{aligned} $$
From equation \((1),\) the speed of the fluid in the left end of the main pipeis
$$ \mathrm{p}_{1}+\frac{1}{2} \rho v_{1}^{2}=\mathrm{p}_{2}+\frac{1}{2} \rho v_{2}^{2} $$
$$ \begin{array}{l} \mathrm{P}_{1}+\frac{1}{2} \rho v_{1}^{2}=p_{1}-\rho g\left(h_{1}-h_{2}\right)+\frac{1}{2} \rho\left(\frac{A_{1}}{A_{2}}\right)^{2} v_{1}^{2} \\ \mathrm{p}_{1}+\frac{1}{2} \rho v_{1}^{2}=p_{1}-\rho g\left(h_{1}-h_{2}\right)+\frac{1}{2} \rho\left(\frac{A_{1}}{A_{2}}\right)^{2} v_{1}^{2} \\ \quad \frac{1}{2} \rho v_{1}^{2}=-\rho g\left(h_{1}-h_{2}\right)+\frac{1}{2} \rho\left(\frac{A_{1}}{A_{2}}\right)^{2} v_{1}^{2} \end{array} $$
$$ \begin{aligned} \frac{1}{2} \rho\left(\frac{A_{1}}{A_{2}}\right)^{2} v_{1}^{2}-\frac{1}{2} \rho v_{1}^{2} &=\rho g\left(h_{1}-h_{2}\right) \\ \frac{1}{2} \rho\left(\left(\frac{A_{1}}{A_{2}}\right)^{2}-1\right) & v_{1}^{2}=\rho g\left(h_{1}-h_{2}\right) \\ v_{1}^{2} &=\frac{\rho g\left(h_{1}-h_{2}\right)}{\frac{1}{2} \rho\left(\left(\frac{A_{1}}{A_{2}}\right)^{2}-1\right)} \end{aligned} $$
Therefore the speed is
\(v_{1}=\sqrt{\frac{2 g\left(h_{1}-h_{2}\right)}{\left(\left(\frac{A_{1}}{A_{2}}\right)^{2}-1\right)}}\)
Part A
The gauge pressure is \({\rho g h_{1}}\).
Part B
The speed is
\(v_{1}=\sqrt{\frac{2 g\left(h_{1}-h_{2}\right)}{\left(\left(\frac{A_{1}}{A_{2}}\right)^{2}-1\right)}}\)
Related Solutions
real-world applications of calibration of pressure gauge
1. How deep can a submarine that is able to support a gauge pressure of 20atm...
Does a manometer (with one side open) measure absolute pressure or gauge pressure?
What does the top pressure gauge in the figure read?
1) Why was the Micrococcus at the bottom of the broth tube? 2) What growth pattern(s)...
The air pressure on the bottom of an airplane's wings is greater than the pressure on...
1. What things are important in determining the hydrostatic pressure at the bottom of a liquid...
Saturated steam at a gauge pressure of 0.50 bar is to be used to heat a...
2 cylinders are connected by a horizontal tube at the bottom of each cylinder. Cylinder A...
An ideal gas having a volume of 1.0 liters at 40c and a gauge pressure of...
- C PROGRAMMIMG I want to check if my 2 input is a number or not all...
- In long paragraphs answer the questions below: Discuss the key components (where, when, what) and causes...
- Sinkal Co. was formed on January 1, 2018 as a wholly owned foreign subsidiary of a...
- Larry’s best friend, Garfield, owns a lasagna factory. Garfield’s financial skills are not very strong, so...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago