Question
In: Mechanical Engineering
A tank contains a two-phase liquid–vapor mixture of Refrigerant 22 at 10 bar. The mass of...
A tank contains a two-phase liquid–vapor mixture of
Refrigerant 22 at 10 bar. The mass of saturated liquid in the tank
is 25 kg and the quality is 30%.
Determine the volume of the tank, in m3, and the percentage of
the total volume occupied by saturated vapor.
Solutions
Expert Solution
Using Table A-8 (Saturated R22: Pressure Table);
- at P = 10 bar;
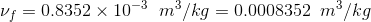
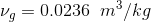
Specific volume at x = 30% = 0.30 is given as;
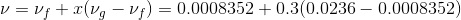
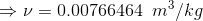
Total mass is given as;
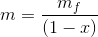
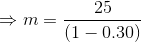
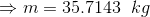
Total volume of the tank;
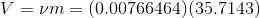
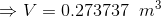
...(Answer)
Mass of saturated vapor;
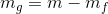
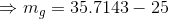
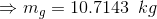
Volume of saturated vapor;
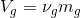
Percentage of total volume occupied by saturated vapor;
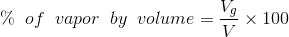
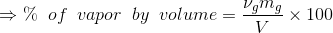
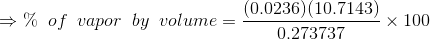
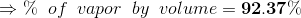
...(Answer)
Related Solutions
A closed, rigid tank contains a two-phase liquid–vapor mixture of Refrigerant 22 initially at -20°C with...
A closed, rigid tank contains a two-phase liquid–vapor mixture
of Refrigerant 22 initially at -20°C with a quality of 47.50%.
Energy transfer by heat into the tank occurs until the refrigerant
is at a final pressure of 6 bar.
a) Determine the final temperature, in °C.
b) If the final state is in the superheated vapor region, at what
temperature, in °C, does the tank contain only saturated vapor?
A two-phase liquid–vapor mixture of Refrigerant 134a is contained in a 2-ft3, cylindrical storage tank at...
A two-phase liquid–vapor mixture of Refrigerant 134a is
contained in a 2-ft3, cylindrical storage tank at 100 lbf/in.2
Initially, saturated liquid occupies 1.6 ft3. The valve at the top
of the tank develops a leak, allowing saturated vapor to escape
slowly. Eventually, the volume of the liquid drops to 0.6 ft3. If
the pressure in the tank remains constant, determine (a) the mass
of refrigerant that has escaped, in lb, and (b) the heat transfer,
in Btu.
PLEASE USE THE...
Determine the volume, in ft3, of 2 lb of a two-phase liquid–vapor mixture of Refrigerant 134A...
Determine the volume, in ft3, of 2 lb of a two-phase
liquid–vapor mixture of Refrigerant 134A at 44°F with a quality of
40%.
A rigid, well-insulated tank contains a two-phase mixture consisting of 0.005 ft3 of saturated liquid water...
A rigid, well-insulated tank contains a two-phase mixture
consisting of 0.005 ft3 of saturated liquid water and 2.7 ft3 of
saturated water vapor, initially at 14.7 lbf/in.2 A paddle wheel
stirs the mixture until only saturated vapor remains in the tank.
Kinetic and potential energy effects are negligible. For the water,
determine the amount of energy transfer by work, in Btu.
A 10-ft3 tank contains a saturated mixture of refrigerant R-134a at a pressure of 40 psia....
A 10-ft3 tank contains a saturated mixture of refrigerant R-134a
at a pressure of 40 psia. If the saturated liquid occupies 2% of
the volume, determine the a) total mass (lbm) b) quality c) average
internal energy (Btu lbm) d) temperature (℉).
A rigid 10-L vessel initially contains a mixture of liquid water and vapor at 100C with...
A rigid 10-L vessel initially contains a mixture of liquid water
and vapor at 100C with 12.3 percent quality. The mixture is then
heated until its temperature is 180C. Calculate the heat transfer
required for this process.
A vapor-compression refrigeration cycle working with R22 contains a liquid-to-suction heat exchanger. The saturated liquid refrigerant...
A vapor-compression refrigeration cycle working with R22
contains a liquid-to-suction heat exchanger. The saturated liquid
refrigerant at 40 °C leaving the condenser and entering the heat
exchanger is used to superheat the saturated vapor refrigerant
leaving the evaporator at 7 °C by 8 °C. If the compressor is
capable of pumping 5 l/s of vapor refrigerant measured at the inlet
to the compressor and the compression processes are considered
isentropic in both cases listed below, determine;
(a) The refrigerating capacity...
A supply line carries a two-phase liquid-vapor mixture of steam at 20 bars, state 1. A...
A supply line carries a two-phase liquid-vapor mixture of steam
at 20 bars, state 1. A small fraction of the flow in the line is
diverted through a throttling calorimeter and exhausted to the
atmosphere at 100 kPa. The temperature of the exhaust steam, state
2 is measured as 373 K. Determine (a) the quality at the exit in
%
A mass of 5 kg of saturated liquid vapor mixture of water is contained in a...
A mass of 5 kg of saturated liquid vapor mixture of water is
contained in a piston cylinder device at 100 kPa, initially 2 kg of
water is in the liquid phase and the rest is in the vapor
phase.Heat is now transferred to the water and the piston which is
resting on a set of stops, starts moving when the pressure in side
reaches 200 kPa, heat transfer continues until the total volume
increases by 20%, determine a. the...
Give an example of a vapor-liquid phase diagram and explain the difference in liquid and vapor...
Give an example of a vapor-liquid phase diagram and explain the
difference in liquid and vapor composition at a given temperature
during the distillation process.
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
- For this assignment you will write a program with multiple functions that will generate and save...
- How many grays is this?Part A A dose of 4.7 Sv of γ rays in a...
- how to operate a business?
ADVERTISEMENT
 samet mamat answered 1 month ago
samet mamat answered 1 month ago