Question
In: Chemistry
Reagent Data Table. Fill in the missing values for each entry. [4] note: if you were...
Reagent Data Table. Fill in the missing values for each entry.
[4] note: if you were unable to determine the yield, you must give an example yield calculation
Table 1. Reagent and Yield Data for the synthesis of butyl acetate from cesium acetate.
Compound Amount MW (g/mol) mmoles Stoichiometry/Comments
acetaminophen 350 mg
ethyl iodide 300 µL
(d = g/mL)
NaOEt 2.6 mL
ethanol 2 mL
phenacetin product obtained: 0.0175 g
crude product yield1 =
recrystallized yield1 =
Solutions
Expert Solution
Ans: The % yields can be calculated as below
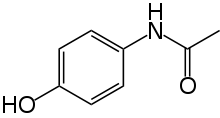 -------->>>
-------->>> 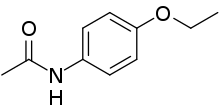
Acetaminophen Phenacetin
151.163 g/mol M.W. 179.219 g/mol
A one mole of acetaminophen reacts with ethyl iodide and NaOEt and give one mole of the Phenacetin.
151.163 g of acetaminophen will give 179.219 g of Phenacetin
Therefore, 1 g acetaminophen will give = 179.219 / 151.163 = 1.1856 g of Phenacetin
So, 350 mg (= 0.350 g) will give = 1.1856 * 0.350 = 0.4150 g of Phenacetin
(Calculated yield based upon the amount of acetaminophen taken)
Practically crude phenacetin obtained = 0.0175 g
% Yield = [Obtained yield (g) / Theoretical (calculated) yield (g)] * 100
% Yield = (0.0175 g / 0.4150 g) * 100 = 4.22 %
Related Solutions
Use the information in the 1st table (Weights & Periods) to fill in the missing values...
Fill in the missing values in the table below given that , dy/dt = 0.8y -...
fill in the missing future values in the following table for an ordinary annuity. Number of...
1) In the first table, there are missing values for direct and cross rates. Please fill...
Calculate and fill in the missing values in the table below Moon Distance from Jupiter Period...
Fill in the missing values for this ANOVA summary table round to two decimal places: S.S....
4. Examine Table 1 below. Fill-in the normal range of values for each of the variables...
Fill in the missing amounts for the following 4 companies. Each case is independent of the...
Cost-Volume-Profit Relations: Fill in Missing Data Following are data from 4 different companies. Provide the missing...
sing Data Set C, fill in the missing data.
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 queen_honey_blossom answered 3 months ago
queen_honey_blossom answered 3 months ago