Question
In: Chemistry
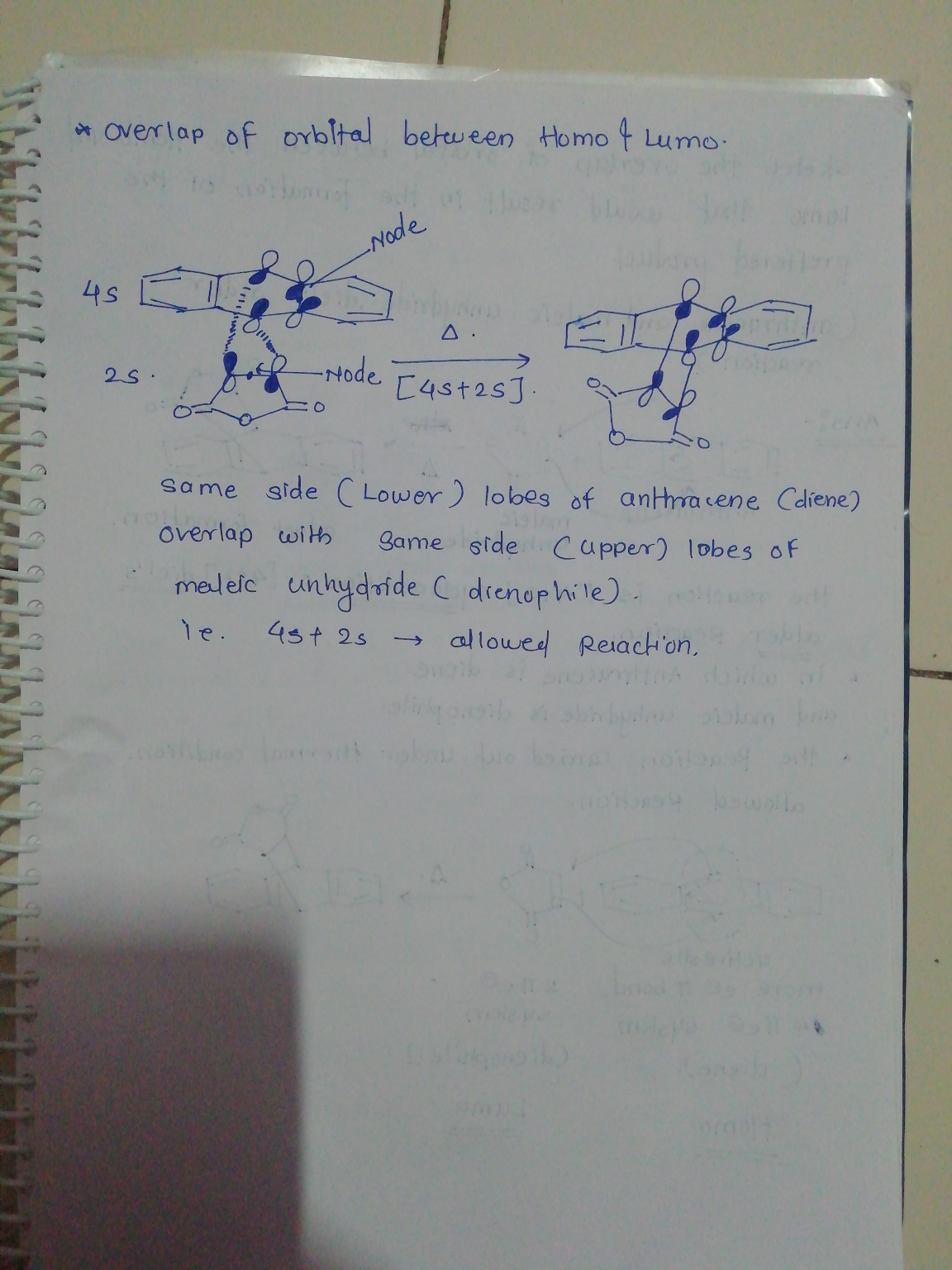
Sketch the overlap of orbitals between the HOMO and LUMO that would result in the formation...
Sketch the overlap of orbitals between the HOMO and LUMO that would result in the formation of the preferred product. (anthracene and maleic anhydride diels-alder reaction)
Solutions
Related Solutions
How do I identify HOMO and LUMO when given orbitals
How do I identify HOMO and LUMO when given orbitals
For a π bond, p orbitals should be [x] when they overlap. As a result, in...
For a π bond, p orbitals should be [x] when
they overlap. As a result, in the E2 mechanism, an
[y] elimination is said to occur.
What type of bond (sigma or pi) will result from the overlap of the following orbitals...
What type of bond (sigma or pi) will result from the overlap of
the following orbitals on adjacent atoms?
a) H(s)-C(sp3)
b) C(sp2)-C(sp3)
c) C(sp3)-N(p)
d) C(p)-O(p)
What type of bond (sigma or pi) will result from the overlap of the following orbitals...
What type of bond (sigma or pi) will result from the overlap of
the following orbitals on adjacent atoms? a) H(s)-C(sp3) b)
C(sp2)-C(sp3) c) C(sp3)-N(p) d) C(p)-O(p)
Illustrate the orbital overlap between the bonding electrons in water. Which two orbitals overlap when a...
Illustrate the orbital overlap between the bonding electrons in
water.
Which two orbitals overlap when a chlorine atom and an iodine
atom overlap to form a covalent bond? Draw an illustration of the
orbital overlap. Are the bonding electrons shared equally between
the two atoms? Explain your reasoning.
Skethc the HOMO diagram of BH3, and the LUMO diagram of BH3NH3 and BH2NH2
Skethc the HOMO diagram of BH3, and the LUMO diagram of BH3NH3
and BH2NH2
Which hybrid orbitals overlap in the C - O bond in CF₂O? Which hybrid orbitals overlap...
Which hybrid orbitals overlap in the C - O bond in
CF₂O? Which hybrid orbitals overlap in the C - O bond in
CF₂O?
A) sp² - s
B) sp² - sp²
C) sp² - sp³
D) sp³ - sp³
E) sp³ - sp
Which orbitals overlap to form the sigma bond between the sulfur and fluorine atoms in the...
Which orbitals overlap to form the sigma bond between the sulfur
and fluorine atoms in the molecule SF4?
Sketch the 5p and 4d orbitals
Sketch the 5p and 4d orbitals
From the output calculations the Homo value is (-7.513) and LUMO is (-3.335) so the calculated...
From the output calculations the Homo value is (-7.513) and LUMO is
(-3.335) so the calculated band gap was 4.17 ev .
According to the following equation :
Wavelength(in nm)=1240.8/Energy(in eV) = 297.55 nm
My question is why I got this small wavelength 297.55 mm while
the maximum wavelength from UV-VIS was above 500 mm ??
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
ADVERTISEMENT


 queen_honey_blossom answered 3 months ago
queen_honey_blossom answered 3 months ago