Question
In: Chemistry
Which hybrid orbitals overlap in the C - O bond in CF₂O? Which hybrid orbitals overlap...
Which hybrid orbitals overlap in the C - O bond in
CF₂O? Which hybrid orbitals overlap in the C - O bond in
CF₂O?
A) sp² - s
B) sp² - sp²
C) sp² - sp³
D) sp³ - sp³
E) sp³ - sp
Solutions
Expert Solution
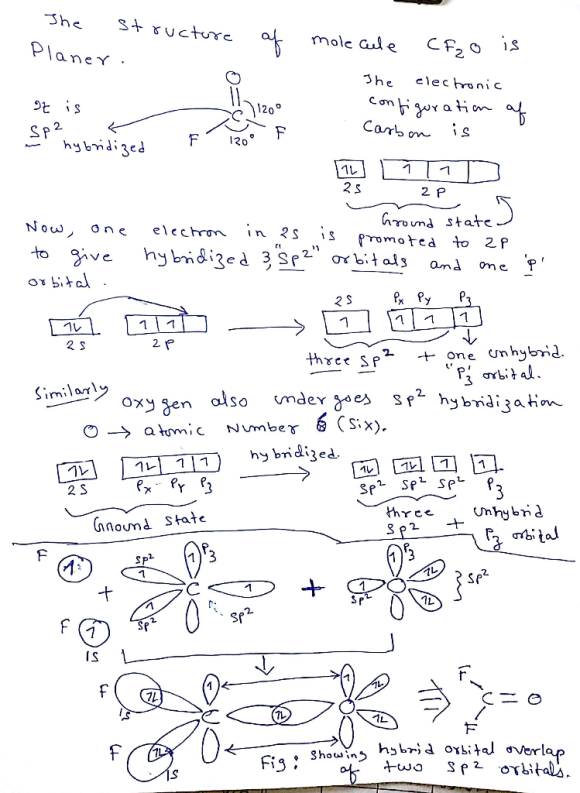 Figure: Showing the overlap of
two SP2 hybrid orbital.
Figure: Showing the overlap of
two SP2 hybrid orbital.
Hence the answer is option B.SP2--SP2
Related Solutions
What atomic or hybrid orbitals make up the sigma bond between C2 and O in acetaldehyde,...
What atomic or hybrid orbitals make up the sigma bond between
C2 and O in
acetaldehyde, CH3CHO
?
(C2 is the second carbon in the structure as
written.)
orbital on
C2
+
orbital on
O
What is the approximate
C1-C2-O
bond angle ? ... °
What atomic or hybrid orbitals make up the pi bond between N and O in nitrosyl...
What atomic or hybrid orbitals make up the pi bond between N and
O in nitrosyl bromide, NOBr ?
________ orbital on N + ____________ orbital on O.
How many σ bonds does N have in NOBr ?
How many pi bonds does N have ?
Which orbitals overlap to form the sigma bond between the sulfur and fluorine atoms in the...
Which orbitals overlap to form the sigma bond between the sulfur
and fluorine atoms in the molecule SF4?
9.) Describe a sigma bond. A) overlap of two f orbitals B) end to end overlap...
9.) Describe a sigma bond.
A) overlap of two f orbitals
B) end to end overlap of p orbitals
C) s orbital overlapping with the side of a p orbital
D) side by side overlap of p orbitals
E) p orbital overlapping with a d orbital
10.) What is the molecular geometry of SCl4?
A) tetrahedral
B) seesaw
C) square pyramidal
D) square planar
For a π bond, p orbitals should be [x] when they overlap. As a result, in...
For a π bond, p orbitals should be [x] when
they overlap. As a result, in the E2 mechanism, an
[y] elimination is said to occur.
Use valance bond theory to describe which atomic orbitals overlap in N2. Draw a bonding scheme...
Use valance bond theory to describe which atomic orbitals
overlap in N2. Draw a bonding scheme that shows the atomic orbitals
overlapping.
Illustrate the orbital overlap between the bonding electrons in water. Which two orbitals overlap when a...
Illustrate the orbital overlap between the bonding electrons in
water.
Which two orbitals overlap when a chlorine atom and an iodine
atom overlap to form a covalent bond? Draw an illustration of the
orbital overlap. Are the bonding electrons shared equally between
the two atoms? Explain your reasoning.
What type of bond (sigma or pi) will result from the overlap of the following orbitals...
What type of bond (sigma or pi) will result from the overlap of
the following orbitals on adjacent atoms?
a) H(s)-C(sp3)
b) C(sp2)-C(sp3)
c) C(sp3)-N(p)
d) C(p)-O(p)
What type of bond (sigma or pi) will result from the overlap of the following orbitals...
What type of bond (sigma or pi) will result from the overlap of
the following orbitals on adjacent atoms? a) H(s)-C(sp3) b)
C(sp2)-C(sp3) c) C(sp3)-N(p) d) C(p)-O(p)
Use valence bond theory to describe the number and types of hybrid bonding orbitals on the...
Use valence bond theory to describe the number and types of
hybrid bonding orbitals on the central atom of each of the
following.
(a) AlF4− type of hybridization:
sp-hybridization
sp2-hybridization
sp3-hybridization
sp3d-hybridization
sp3d2-hybridization
In AlF4−, aluminum has hybrid
orbital(s).
(b) IBr3
type of hybridization:
sp-hybridization
sp2-hybridization
sp3-hybridization
sp3d-hybridization
sp3d2-hybridization
In IBr3, iodine has hybrid orbital(s).
(c) CS2
type of hybridization:
sp-hybridization
sp2-hybridization
sp3-hybridization
sp3d-hybridization
sp3d2-hybridization
In CS2, carbon has hybrid orbital(s).
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
- For this assignment you will write a program with multiple functions that will generate and save...
- How many grays is this?Part A A dose of 4.7 Sv of γ rays in a...
ADVERTISEMENT
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago