Question
In: Physics
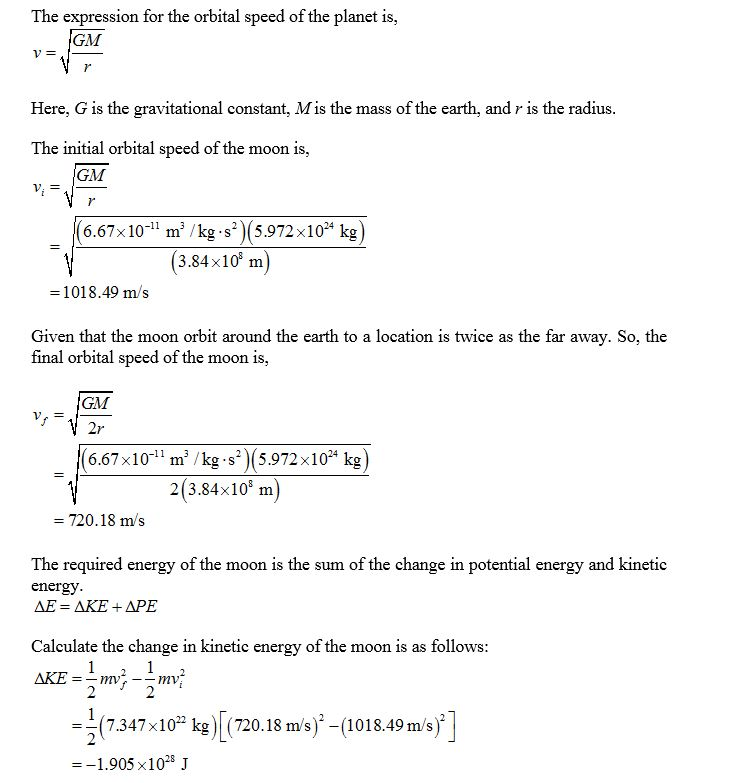
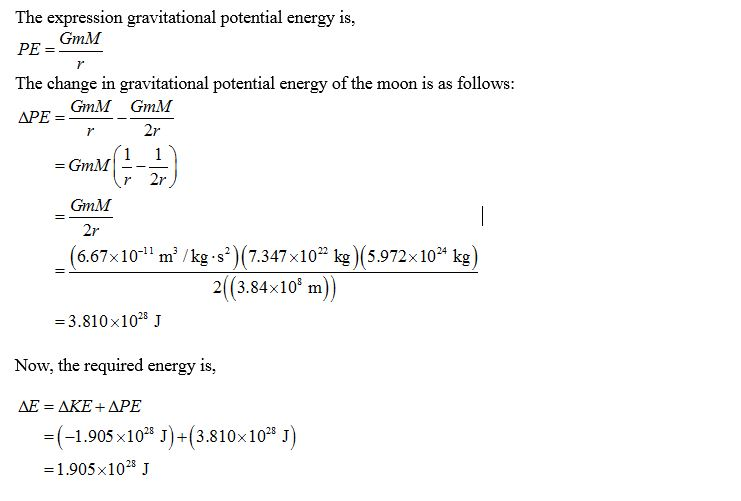
How much energy Δ? would be required to move the Moon from its present orbit around...
How much energy Δ? would be required to move the Moon from its present orbit around Earth to a location that is twice as far away? Assume the Moon’s orbit around Earth is nearly circular and has a radius of 3.84×108 m.
Solutions
Related Solutions
How much energy, in kiloJoules, would be required to raise the temperature of 1.00 kilogram of...
How much energy, in kiloJoules, would be required to raise the
temperature of 1.00 kilogram of liquid benzene
(C6H6) from 20.0 dC to its boling point of
80.1dC and then to completely vaporize the benzene at this same
temperature? The specific heat capacity of benzene is 1.74J
g-1 0C-1. The molar enthalpy of vaporization
of benzene is 1.929 kJ/mole.
What is Earth's Specific Mechanical Energy in its orbit around the Sun? Here are some properties...
What is Earth's Specific Mechanical Energy in its orbit around
the Sun?
Here are some properties you may or may not need to solve this
question (you'll have to decide which of these will help you):
Earth's semi-major axis in its orbit around the sun, a = 149.6 x
106 km
Earth's mass is 6 x 1024 kg
Earth's Gravitational Parameter, μEarth = 3.986 x 105 km3
s-2
Sun's Gravitational Parameter, μSun = 1.327×1011 km3 s-2
Sun's mass is 2...
How much energy is required to raise the temperature of 100g of water from -10 to...
How much energy is required to raise the temperature of 100g of
water from -10 to 110°C?
Why does the moon have less gravity and how does the moon stay in orbit?
Why does the moon have less gravity and how does the moon stay
in orbit?
Planet X has a moon that has a very elliptical orbit. Its furthest point from the...
Planet X has a moon that has a very elliptical orbit. Its
furthest point from the planet X is 4 × 10^8 m and its closest
point is 3 × 10^8 m. If its speed at the furthest point is 700 m/s,
what is its speed at its closest point?
The mass of planet X is 2.68 X 10^24 kg and the radius of planet
X is 4079 km. (please show work and drawing)
How much energy is required to heat 1.5 kg of frozen water from -10
How much energy is required to heat 1.5 kg of frozen water
from -10
What factors affect the speed of a satellite in circular orbit around Earth. And, how would...
What factors affect the speed of a satellite in circular orbit
around Earth. And, how would you calculate that speed?
A hollow hoop and a solid disk are released from rest at the top
of an incline plane that has friction. Which will reach the bottom
of the hill first and why?
What is impulse and how does it relate to the average force
applied to an object during a collision? Include one physical
example in your explanation.
1. How much energy is required to change a 41 g ice cube from ice at...
1. How much energy is required to change a 41 g ice cube from
ice at −14◦C to steam at 115◦C? The specific heat of ice is 2090
J/kg · ◦ C, the specific heat of water is 4186 J/kg · ◦ C, the
specific heat of stream is 2010 J/kg · ◦ C, the heat of fusion is
3.33 × 105 J/kg, and the heat of vaporization is 2.26 × 106 J/kg.
Answer in units of J.
How much heat energy is required to warm up 2.35 kg of water, from 20oC to...
How much heat energy is required to warm up 2.35 kg of water,
from 20oC to 83oC ? Need to show unit conversion and all referents
in calculation.
Ionization involves completely removing an electron from an atom. Part A How much energy is required...
Ionization involves completely removing an electron from an
atom.
Part A
How much energy is required to ionize a hydrogen atom in its
ground (or lowest energy) state?
E =
J
Part B
What wavelength of light contains enough energy in a single
photon to ionize a hydrogen atom?
λ =
nm
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
ADVERTISEMENT
 genius_generous answered 3 months ago
genius_generous answered 3 months ago