Question
In: Chemistry
The energy of light photons varies with wavelength. Calculate the energy per mole of photons for...
The energy of light photons varies with wavelength. Calculate the energy per mole of photons for each of the following colors of visible light. Red Light, ? = 691 nm Green Light, ? = 527 nm Blue Light, ? = 411 nm
Solutions
Expert Solution
Part 1)
Red light, 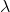 = 691 nm
= 691 nm
Frequency, v = c/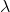 = (3 x 108 m/s)/(691 x 10-9 m ) = 4.341 x 1014/s
= (3 x 108 m/s)/(691 x 10-9 m ) = 4.341 x 1014/s
Energy, E = hv = (6.626 x 10-34 J s) x (4.341 x 1014 /s) = 2.876 x 10-19 J
Energy per mol = (2.84 x10-19 J) x (6.023 x 1023 photons/mol) = 1.732 x 105 J/mol = 1.732 x 102 kJ/mol
Energy of red light = 1.73 x 102 kJ/mol
Part 2)
Green Light, 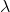 = 527 nm
= 527 nm
Frequency, v = c/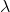 = (3 x 108 m/s)/(527 x 10-9 m ) = 5.692 x
1014/s
= (3 x 108 m/s)/(527 x 10-9 m ) = 5.692 x
1014/s
Energy, E = hv = (6.626 x 10-34 J s) x (5.692 x 1014/s) = 3.771 x 10-19 J
Energy per mol = (3.771 x 10-19 J) x (6.023 x 1023 photons/mol) = 2.271 x 105 J/mol = 2.271 x 102 kJ/mol
Energy of green light = 2.27 x 102 kJ/mol
Part 3)
Blue Light, 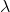 = 411 nm
= 411 nm
Frequency, v = c/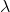 = (3 x 108 m/s)/(411 x 10-9 m ) = 7.299 x
1014/s
= (3 x 108 m/s)/(411 x 10-9 m ) = 7.299 x
1014/s
Energy, E = hv = (6.626 x 10-34 J s) x (7.299 x 1014/s) = 4.836 x 10-19 J
Energy per mol = (4.836 x 10-19 J) x (6.023 x 1023 photons/mol) = 2.913 x 105 J/mol = 2.913 x 102 kJ/mol
Energy of blue light = 2.91 x 102 kJ/mol
Related Solutions
The energy of light photons varies with wavelength. Calculate the energy per mole of photons for...
What is the energy per mole of photons of light with a wavelength of 812.4 nm?...
Calculate the wavelength ?λ and the frequency ?f of the photons that have an energy of...
What is the energy, in kJ/mole, associated with photons having the following wavelength A. 280nm B....
calculate the energy of red light with a wavelength of 703.2 nm
One photon of a certain light has an energy of 3.8e-19J. Calculate the wavelength of the light.
Be sure to answer all parts. Calculate the wavelength (in nm) of light with energy 1.89...
What is the electromagnetic spectrum? What are the wavelength ranges of light? What are photons?
6.a) In terms of wavelength, frequency and energy compare the two photons with wavelengths of 900...
Determine the energy of 1.90 mol of photons for each of the following kinds of light....
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
 queen_honey_blossom answered 4 months ago
queen_honey_blossom answered 4 months ago