Question
In: Chemistry
Describe how PPV is made and how it acts as a light emitting diode. Describe how...
- Describe how PPV is made and how it acts as a light emitting diode.
- Describe how its structure can be altered to change the color of light emitted by the LED.
- Summarize the benefits and drawbacks of OLEDS over inorganic semiconductors.
Solutions
Expert Solution
Poly(p-phenylene vinylene) (PPV, or polyphenylene vinylene) is a conducting polymer of the rigid-rod polymer family. PPV is the only polymer of this type that can been processed into a highly ordered crystalline thin film. PPV and its derivatives are electrically conducting upon doping. Although insoluble in water, its precursors can be manipulated in aqueous solution. The small optical band gap and its bright yellow fluorescence makes PPV a candidate in applications such as light-emitting diodes (LED) and photovoltaic devices.
PREPARATION
PPVs can be synthesized by a variety of methods, the details of which determine purity and molecular weight. The most popular methods proceed via p-xylylene intermediates after a base induced elimination from α,α'-disubstituted para-xylenes.
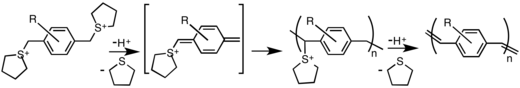
Although xylylene-based routes dominate the synthetic methodology, many other routes have been evaluated.
Step growth routes: PPV can be
synthesized by Wittig-type couplings between the bis(ylide) derived
from an aromatic bisphosphonium salt and dialdehyde, especially
1,4-benzenedialdehyde. 
Step growth coupling reactions, such as this Wittig condensation, usually yield low molecular weight oligomer with 5-10 repeat units. Incorporation of various side groups (alkyl, alkoxy, or phenyl) increases the solubility of the polymer and gives higher molecular weights. An advantage of the step-polymerization approach is that ortho-, meta-, and para-xylylene linkages can be incorporated in the main chain. Copolymers of defined stereoregularity can also be easily made in this way.
PPV derivatives can be also produced via the Knoevenagel
condensation between a benzylic nitrile and an aromatic dialdehyde.
Since this method produces many side reactions, such as hydrolysis
of nitrile group, careful optimization of the reaction conditions
was needed. 
Heck coupling routes:The
couplings of ethylene with a variety of aromatic dibromides via a
Heck reaction give reasonable molecular weights (3,000-10,000) when
solubilizing groups present. However, this method requires one of
the gaseous starting materials to be added in precise amounts, In
excess polyethylene could be formed. 
Ring-opening routes: A bicyclooctadiene compound has been coupled by ring-opening metathesis polymerization (ROMP) to give a precursor polymer of high molecular weight and soluble in organic solvents. This polymer can be deposited as thin films and converted thermally to PPV. Lower conversion temperatures could be employed with the presence of an amine catalyst.
AS LIGHT- EMIITING DIODE
Polyphenylene vinylene is capable of electroluminescence, leading to applications in polymer-based organic light emitting diodes. PPV was used as the emissive layer in the first polymer light-emitting diodes.[6] Devices based on PPV emit yellow-green light, and derivatives of PPV obtained by substitution are often used when light of a different color is required. In presence of even a small amount of oxygen, singlet oxygen is formed during operation, by energy transfer from the excited polymer molecules to oxygen molecules. These oxygen radicals then attack the structure of the polymer, leading to its degradation. Special precautions therefore have to be kept during manufacturing of PPV in order to prevent oxygen contamination.
Related Solutions
10.How does a light-emitting diode work? Why are there different colors of LEDs?
Consider a diffraction grating with 600 lines per millimeter. Light from a light–emitting diode at λ...
Light emitting diode (LED) light bulbs have become required in recent years, but do they make...
Light emitting diode (LEDs) light bulbs have become required in recent years, but do they make...
Light emitting diode (LEDs) light bulbs have become required in recent years, but do they make...
Light emitting diode (LED) light bulbs have become required in recent years, but do they make...
Light-emitting diode (LED) light bulbs have become required in recent years, but do they make financial...
Payback Period and IRR of a Cost Reduction Proposal-Differential Analysis A light-emitting diode (LED) is a...
The city of Metropolis is considering replacing incandescent bulbs in traffic lights with light-emitting diode (LED)...
Light emitting diodes, lasers 1. Describe what physical property of a semiconductor will deem it good...
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
 queen_honey_blossom answered 9 months ago
queen_honey_blossom answered 9 months ago