Question
In: Chemistry
How do you find the rate constant here? trials [I] [H2O2] [H] temp (C) Rate (M/s)...
How do you find the rate constant here?
| trials | [I] | [H2O2] | [H] | temp (C) | Rate (M/s) |
| 1 | 8.53*10^-3 | 1.03*10^-1 | 0.333 | 21.5 | 5.33*10^-4 |
| 2 | 8.53*10^-3 | 1.03*10^-1 | 0.333 | 15 | 4.3*10^-4 |
| 3 | 8.53*10^-3 | 1.03*10^-1 | 0.333 | 42 | 2.17*10^-3 |
Solutions
Expert Solution
In chemical kinetics a reaction rate constant,
k or 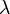 , quantifies the rate of a chemical reaction.[1]
, quantifies the rate of a chemical reaction.[1]
For a reaction between reactants A and B to form product C

the reaction rate is often found to have the form:

Here k(T) is the reaction rate constant that depends on temperature. [A] and [B] are the concentrations of substance A in moles per volume of solution assuming the reaction is taking place throughout the volume of the solution. (For a reaction taking place at a boundary one would use instead moles of A or B per unit area).
| trials | [I] | [H2O2] | [H] | temp (C) | Rate (M/s) |
| 1 | 8.53*10^-3 | 1.03*10^-1 | 0.333 | 21.5 | 5.33*10^-4 |
| 2 | 8.53*10^-3 | 1.03*10^-1 | 0.333 | 15 | 4.3*10^-4 |
| 3 | 8.53*10^-3 | 1.03*10^-1 | 0.333 | 42 | 2.17*10^-3 |
1.  T is
in K
T is
in K
on solving we get k= 6.827*10-3 L2M-1s-1
2. 
on puttin values and solving
k = 6.981*10-3 L2M-1s-1
3. 
on puttin values and solving
k = 6.383*10-3 L2M-1s-1
Related Solutions
How do I calculate the rate law constant and find the overall rate law?
a.) The rate constant for the reaction is 0.460 M–1·s–1 at 200 °C. A--> products. If...
a.) The rate constant for the reaction is 0.460 M–1·s–1 at 200 °C. A--> products. If...
A second-order reaction has a rate constant of 0.009500/(M · s) at 30°C. At 40°C, the...
The rate constant for the reaction is 0.130 M–1·s–1 at 200 °C. If the initial concentration...
How do you solve the molarity of H2O2 after mixing? There are 2 mL of H2O2,...
How do you find the spot rate and forward rate of the Japanese Yen? I need...
C++. How do I reverse this encryption? Here is the question: "A company that wants to...
A) A car travels at a constant speed of 32.5 mi/h (14.5 m/s) on a level...
how to implement h / k sqrt(n) in c h being a constant k being the...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 queen_honey_blossom answered 10 months ago
queen_honey_blossom answered 10 months ago