Question
In: Chemistry
1. The dipole moment of BrCl is 0.518 D and the distance between atoms is 213.9 pm....
1. The dipole moment of BrCl is 0.518 D and the distance between atoms is 213.9 pm. What is the percent ionic character of the BrCl bond?
Solutions
Expert Solution
When a covalent bond is formed between two atoms of different electronegativities, the electrons of the covalent bond are not shared equally between the two atoms. Such a bond is known as polar covalent bond.
Due to this polar nature of the covalent bond a dipole is generated.
The dipole moment of a molecule is the measure of the polarity or the separation of the charge of a covalent bond.
Given:
Observed Dipole moment of BrCl: μObs = 0.518 D
Distance between the atoms: d = 213.9 pm = 213.9 x 10-12 m (since, 1pm = 10-12 m)
Also,
Charge = 1.6 x 10-19 C
Now, the dipole moment of BrCl bond is given by the equation,
Dipole moment (μCal) = Charge (q) x Distance between
the atoms (d)
= (1.6 x 10-19 C) x (213.9 x 10-12 m)
= 342.24 x 10 -31 Cm = 3.4224 X 10-29 Cm
Since, 3.33564×10−30C·m = 1 D
Therefore, 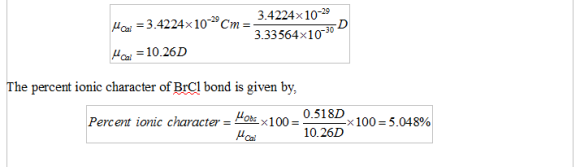
Related Solutions
What is the difference between a magnetic dipole and a magnetic moment?
The ammonia molecule NH3 has a permanent electric dipole moment equal to 1.47 D, where 1...
A hypothetical molecule, X–Y, has a dipole moment of 1.96 D and a bond length of...
IF5 Polar Bonds Yes No Dipole Moment Yes No TeBr4 Polar Bonds Yes No Dipole Moment...
Identify whether each of the following compounds exhibits a molecular dipole moment. If so, indicate the direction of the net molecular dipole moment:
What is the relationship between distance in nanometers and the number of atoms? What is the...
What is the formula to calculate the dipole moment of a magnet? This is the information...
What is the measured component of the orbital magnetic dipole moment of an electron with (a)...
What is the measured component of the orbital magnetic dipole moment of an electron with (a)...
What is the measured component of the orbital magnetic dipole moment of an electron with (a)...
- In long paragraphs answer the questions below: Discuss the key components (where, when, what) and causes...
- Sinkal Co. was formed on January 1, 2018 as a wholly owned foreign subsidiary of a...
- Larry’s best friend, Garfield, owns a lasagna factory. Garfield’s financial skills are not very strong, so...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
 queen_honey_blossom answered 1 year ago
queen_honey_blossom answered 1 year ago