Question
In: Chemistry
Consider the followimg techniques and explain completely why the later eluting peaks in a simple chromatogram...
Consider the followimg techniques and explain
completely why the later eluting peaks in a simple chromatogram are
broader than those eluting at earlier retention times.
a. HPLC conventional
b. UHPLC
c. GC, packed column
d. GC capillary/ WCOT column
e. SFC
Solutions
Expert Solution
GC DETECTORS
The eluent from the column is directed to one or more detectors. These produce signals which are proportional to either the amount of sample present in the detector at any moment, or the concentration of sample in the detector. The detector signal is usually displayed as a plot of signal magnitude versus time, giving the classic chromatogram. Detectors vary in their response to different classes of compound, from the thermal conductivity detector, which is universally responsive to all compounds, to such specialized detectors as the flame photometric detector, which detects only sulfur or phosphorous containing compounds, depending on the way it is set up. The selectivity of a detector is usually expressed by the ratio of the response to the desired analyte divided by that towards an interfering compound. For instance, the selectivity of a sulfur specific detector may be given by its response to a nanogram of sulfur divided by that for a nanogram of hydrocarbon. Since the interfering material may be present in much greater quantity than the analyte, selectivity factors of 104 or more are desirable.
The characteristics sought in a gas chromatographic detector are high sensitivity, a linear dynamic range of 4 orders of magnitude or more, a favorable signal to noise ratio, and good long term stability. A small detector dead volume is also important. If the sample has an opportunity to mix with a volume of carrier gas before the detection process is completed, the peak will be broadened and efficiency lost.
HPLC Columns:
Precolumns and Guard Columns
The analytical column is expensive and can be damaged by particulate material depositing at the head of the column, as well as by attack of the eluent on the packing itself. Eluents with pH outside the 2 to 7 range may dissolve the silica support of the column. To lengthen the useful column life, guard and precolumns are used.
Precolumns are short segments of tubing packed roughly with similar material to that used in the column. The precolumn will pick up any particulates which are present at the exit of the pump. These particles may arise from poorly filtered eluents, or from wear fragments from the pump. More importantly, if the eluent is aggressive, and is dissolving the silica backbone of the packing, the precolumn serves to saturate the eluent with silica. Since it is located before the injector port, it cannot contribute to band broadening. Therefore, it is not necessary to have this column packed as carefully as an analytical column, or to be concerned about having very low dead volume fittings used to install it. It can be made of rather inexpensive components and packed in the lab.
Another source of particulate matter which may damage columns is the sample. To protect the column from materials in the sample which may deposit at the entrance of the column or which may be irreversibly adsorbed on the column packing, a guard column may be used. Guard columns can contribute to loss of separation efficiency because the sample passes through them. Therefore, they must be packed as carefully as the analytical column, and connected with low dead volume fittings. These columns are placed immediately before the analytical column, and can be considered to be the first part of the analytical column. Guard columns are commercially available, usually in 2-5 cm lengths. Some column systems are available which allow a replaceable cartridge to be placed in the inlet fitting of the analytical column, to serve as a guard column.
Eluents:
The choice of eluent depends on the column and the sample. In reverse phase chromatography, a more polar eluent will move the sample slowly, and allow time for separation. A less polar solvent will elute late peaks more quickly and prevent excessive band broadening. There are several measures of eluent strength, including the polarity index, P’. A higher value of P’ indicates a more polar eluent. Often solvents are mixed to produce an eluent of a suitable strength for a particular separation. For instance, various mixtures of methanol and water are used to produce a variety of different polarities, with an increase in the water content making a less strong, more polar eluent for reverse phase work. The polarity index of a solvent mixture Pm composed of solvents ‘a’ and ‘b’ is computed as:
Pm = Pa * xa + Pb * xb
where Pa and Pb are the polarity indexes of a and b, and xa and xb is their volume fraction. The effect of eluent polarity on the capacity factor k’ of a compound is given by the equation:
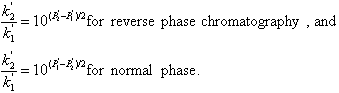
where P1’ and P2’ are the polarity indices of the two eluent mixtures.
The eluent must be able to keep the sample components in solution. The viscosity of the eluent is of concern, because a less viscous solvent can be used at a higher flow, without requiring very high pump pressures. Purity of the eluent, as well as its availability, cost and ease of disposal or recycling are other important considerations. Table Chapter 4 .5 lists some common eluent solvents and their physical characteristics important for HPLC.
Example:
In a reverse phase separation of a pesticide, the retention time was 15.5 min, with an eluent composed of methanol/water at a volume ratio of 30:70. An unretained peak eluted at 0.25 min. Calculate k’.
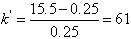
What water/methanol eluent composition will reduce k’ to 5?
Substituting values for methanol and water into Equation 4.20:
P’= 0.3 x 5.1 + 0.7 x 10.2 = 8.7
and 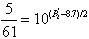 so P2’
=6.52
so P2’
=6.52
To find the composition, let V = volume fraction of methanol.
6.52 = V x 5.51 + (1-V) x 10.2
V = 0.78. Therefore, the eluent is 78% methanol and 22% water.
HPLC DETECTORS:
There is no sensitive universal detector available for use in HPLC. The only really universal, bulk property HPLC detector is the refractive index detector, which cannot be used with gradient elution, requires excellent temperature control, and is as much as 103 times less sensitive than other detectors. Therefore, it finds little use in environmental work. The detectors most often used are those such as absorption spectroscopic detectors, which respond to some property of the sample which is not exhibited by the mobile phase.
Ultraviolet Absorption Detectors
Ultra violet detectors are fairly general in application, since most organic compounds absorb some wavelengths in the UV spectrum. However, the spectral region of wavelengths below 210 is usually not useable for analysis because most solvents which would be used as eluents would also absorb in these areas. The response of this detector depends on Beer's Law, and therefore gives a linear response over four to five orders of magnitude. The detection limits vary widely, depending on the sample component and its extinction coefficient at the wavelength being used. In the most favorable cases, 1 ng or less of a compound may be detected.
Fixed wavelength detectors, using filters to isolate a single band of radiation, are inexpensive and stable. Light is passed through the filter then through a flow cell containing the effluent from the column. Finally, it is allowed to impinge on a photocell, where the light is measured. Generally, these are single beam instruments, but dual beam systems are possible. They lack versatility, since the only compounds which can be analyzed are those which absorb at the fixed wavelength. However, for standardized, repetitive analyses, these detectors may be ideal since their reproducibility is often slightly better than that of variable wavelength detectors.
Variable wavelength detectors, are, however, much more versatile. These use a continuum source and a monochromator to select the wavelength desired. A manually adjusted grating disperses the light and passes the target wavelength through the flow cell.
Detectors which can rapidly perform a complete scan over a range of wavelengths can give qualitative as well as quantitative information. This can be done with a rapid scanning instrument, but, more commonly a diode array detector is used. The photo diode array (PDA) detector uses an arrangement of diodes positioned so that each diode intercepts a different band of wavelengths. The signal from each diode is recorded, and a spectrum of the effluent at any moment is obtained. This is very useful in confirming identity of components, and even more, in determining the efficiency of separation. it,s shows the basic layout of a diode array detector.
The purity of a peak may be determined. Co-elution of components can be confirmed or ruled out by comparing spectra taken on the leading edge, the top, and the trailing edge of a peak. It is difficult to identify a totally unknown compound from the UV spectrum. These are relatively simple spectra, and the solvent, mixed with the sample, has an effect on the spectrum. However, comparison of samples and standards run in the same solvent, gives retention time and spectral information, and strong identification confirmation. Figure Chapter 4 .27 shows part of a chromatogram of a sample of polynuclear aromatic hydrocarbons run using a PDA. The peaks which have spectra and retention times matching those of the standard are identified, and the spectrum of each peak is printed on the report.
The flow cells used for absorption detectors are designed to give the maximum length of sample for the light to pass through, while keeping the volume as small as possible, to insure that the resolution is not compromised. Figure Chapter 4 .28 shows a UV detector cell. The cell has a Z shape to provide the maximum path length with as little cell volume as possible. The principal source of noise in absorbance detectors using a flowing sample is due to slight changes in refractive index. These are due to slight inhomogeneities in the composition of the eluent, changes in temperature, or turbulence in the flow. The change in refractive index diverts some of the light from the path to the detector, momentarily decreasing the signal.
SUPERCRITICAL FLUID CHROMATOGRAPHY
A substance cannot exist in the liquid state at a temperature above its critical temperature. However, if a material is above its critical temperature, and is subjected to sufficiently high pressure, it becomes much more dense than ordinary gases, and takes on some liquid-like properties. This is then referred to as a supercritical fluid. Figure Chapter 4 .32 shows the phase diagram for CO2, a commonly used supercritical fluid. The properties of supercritical fluids can be continuously varied between those of the gas and those of the liquid by changing the temperature and pressure. These fluids can be used as mobile phases in chromatography. Properties which can be varied include the viscosity, solvent properties and diffusivity, all of which are important chromatographic properties.
The properties of these fluids are usually closer to those of liquids than gases. The solubilizing power of a supercritical fluid is much greater than that of a gas. Therefore, nonvolatile and slightly volatile compounds may be separated by supercritical chromatography, while this would be impossible to do with GC. There is also an advantage over HPLC analysis for these compounds, since the solute diffusion coefficients in supercritical fluids are much greater. This means that the eluent velocity required for the maximum column efficiency is 5 to 10 times greater than that for HPLC. Equally efficient separations can therefore be done in much less time than is needed for HPLC. Finally, the viscosity of these fluids is much lower than that of liquids, making them much easier to pump through columns at a faster flow. Both packed and open tubular columns are used.
Any substance stable above its supercritical temperature might be used for eluent in SFC, but only a few are used routinely. Supercritical fluids which have been used are carbon dioxide, nitrous oxide, sulfur hexafluoride, Freon-13, ethane and ammonia. Of these, CO2 is the most common, since its critical temperature, 31oC, is readily attained, it is non-toxic, and is readily available. Between the pressures of 72 and 400 atmospheres, and temperatures of 40 to 140 oC, the density of CO2 can be varied from 0.1 to almost 1 mg/ml. The only practical supercritical fluid which is reasonably polar is ammonia. It is, however, quite reactive and difficult to use. Modifiers are therefore used to improve the separation of more polar substances in nonpolar supercritical fluids such as CO2. Modifiers are added to the eluent to improve peak shape and shorten retention times. These modifiers, including methanol, water and formic acid, are used in low concentrations, below 2% by volume. Modifiers at this low level may be thought of as deactivating silanol groups on the column, rather than increasing the solubility of the sample compounds in the solvent.
Programming in SFC is quite flexible, since temperature, pressure and density all affect the retention of samples. The most common method of programming is density or pressure programming although temperature programming has also been used.
SFC Instrumentation:
The components of an SFC system are similar to those of HPLC, since a pump is used to produce the high pressures required, but GC and HPLC detectors can be used. A typical system is shown in Figure Chapter 4 .33.
A syringe pump is the most commonly used pump although reciprocating pumps have been used for packed column work. The mixing of modifiers complicates the system. Cylinders of eluent with modifier already mixed may be purchased, but then the amount of modifier cannot be adjusted. A second pump to add modifier is useful.
Samples are injected into the system using high pressure rotary sampling valves. Sample volumes as small as a few nanoliters are needed for capillary work, so sample splitting techniques may be required. However, the sample is injected at room temperature, where the eluent may not be supercritical. The sample also may not be homogeneously mixed into the eluent quickly enough before the split is made. Therefore, the operation of a splitter is not always simple, and quantitation may be poor. Sample volumes for packed columns are larger, in the microliter range, so injection is a much easier task.
Columns used in SFC are usually small bore columns packed with 5 to 10 mm bonded phase particles similar to those used for HPLC. Short capillary columns of 1 to 10 meters in length are also used, with stationary phases which are often more crosslinked than those used in GC. All bonded stationary phases have some contribution from unreacted silanol groups, and these can be a problem in SFC, because of the relatively nonpolar nature of CO2. This is why polar modifiers are effective.
At the end of the column a restrictor is required to keep the fluid in the column at the required pressure. The restrictor is positioned before the detector when the detector is a GC type detector, and after the detector, when an HPLC type detector such as a UV absorption cell, is used. A restrictor may be simply a short length of narrow bore fused silica tubing of 5 to 15 microns i.d. However, the sample may precipitate as fog droplets when the solvent suddenly decompresses at the end of the restrictor. These droplets, when fed into a flame ionization detector, cause signal spikes. This may be avoided by decompressing the eluent more gradually in a tapered or conical restrictor, which is kept warm at the tip so that the sample has a chance to evaporate.
The flame ionization detector is probably the most common detector for SFC, as it is compatible with the usual fluids. While organic modifiers interfere, water and formic acid can be used with FID detectors. The sensitivity of the FID is somewhat lower than its GC counterpart. The UV detector is also often used, especially when wide bore columns or organic modifiers make the FID unsuitable. The volume of the absorbance cell must be very small in SFC, on the order of 50 nanoliters or less, to avoid band broadening when capillary columns are used. Standard HPLC UV cells may be used in packed column work, but modifications may be necessary to allow the cells to be used at the much higher pressures common in SFC.
Related Solutions
1) Why do peaks not occur as a simple line? In other words, why is the...
from the laue condition explain why we can observe maximal peaks in the amplitude of the...
Explain why the development of service industry is later than that of agriculture and manufacturing based...
Explain why the development of service industry is later than that of agriculture and manufacturing based...
1) explain why we see the moon rise 52 minutes later each evening, and 2) explain...
Describe the tools and techniques required for planning communications and explain why each is important for...
List, draw and explain the strengthening mechanisms for metals. Why we are using this techniques?
Describe each of the three compliance techniques, explain why each leads to compliance, and provide an...
Explain why the equation for PV of a perpetuity is so simple as = C/Rate, if...
Consider the simple regression model: Yi = β0 + β1Xi + e (a) Explain how the...
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago