Question
In: Physics
calculate the coefficient of isothermal compressibility of a gas at 440 psia and temperature of 80...
calculate the coefficient of isothermal compressibility of a gas at 440 psia and temperature of 80 F. Specific gravity of gas is 0.697. Please show all work.
Solutions
Expert Solution
Thermodynamically, Isothermal compressibility is defined as the reciprocal of Isothermal Bulk modulus of elasticity.
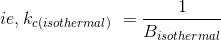
So we have to find Bisothermal as the first step.
Bulk modulus is defined as the ratio between pressure increase and the resulting decrease in a material's volume
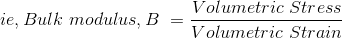
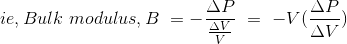
For an Ideal gas, PV = nRT
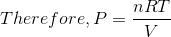
Differentiate P with respect to V keeping temperature constant,
ie isothermal ( )
)
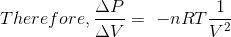
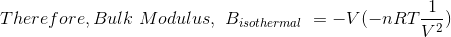
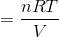
where nRT = PV
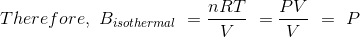
we know that
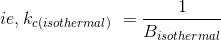
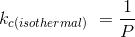
Given P = 440psia = 3.03 x 106 Pa (SI unit)
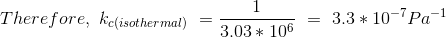
Related Solutions
What is the heat capacity (or specific heat), isothermal compressibility, and coefficient of thermal expansion of...
What is the heat capacity (or specific heat), isothermal
compressibility, and coefficient of thermal expansion of an ideal
gas, van der Waals fluid, electromagnetic field, rubber band, and a
magnetic spin system?
Derive an expression for the reversible isothermal work done on n moles of gas at temperature...
Derive an expression for the reversible isothermal work done on
n moles of gas at
temperature T if the volume changes from V1 to V2 and the gas
obeys van der Walls’ equation.
Temperature coefficient.
The temperature coefficient of most of the reactions lies between:
Calculate deltaS total for the isothermal irreversible free expansion of 1.00 mol of ideal gas from...
Calculate deltaS total for the isothermal irreversible free
expansion of 1.00 mol of ideal gas from 8.0 L to 20.0 L at 298
K
Water at 80°F and 20 psia is heated in a chamber by mixing it with saturated...
Water at 80°F and 20 psia is heated in a chamber by mixing it
with saturated water vapor at 20 psia. If both streams enter the
mixing chamber at the same mass flow rate, determine the
temperature and the quality of the exiting stream. Include a heat
loss rate of 200 kW and a net mass flow rate of the combined
streams of 4 lbm/s.
An ideal gas is brought through an isothermal compression process. The 4.00 mol of gas goes...
An ideal gas is brought through an isothermal compression
process. The 4.00 mol of gas goes from an initial volume of
227.5×10−6 m3 to a final volume of 101.0×10−6 m3. If 8890 J is
released by the gas during this process, what are the temperature ?
and the final pressure ?? of the gas?
An ideal gas is brought through an isothermal compression process. The 3.00 mol of gas goes...
An ideal gas is brought through an isothermal compression
process. The 3.00 mol of gas goes from an initial volume of 222.0 ×
10 − 6 m 3 to a final volume of 123.5 × 10 − 6 m 3 . If 7.60 × 10 3
J is released by the gas during this process, what are the
temperature T and the final pressure p f of the gas
Calculate the diffusion coefficient of N2 at 100 Pa, 100 kPa an 20 MPa (temperature T...
Calculate the diffusion coefficient of N2 at 100 Pa,
100 kPa an 20 MPa (temperature T = 20 degrees celsius).
Calculate the temperature T of 40 kg of CO2 gas in a 500 liter vessel at...
Calculate the temperature T of 40 kg of CO2 gas in a 500 liter
vessel at 5 MPa. The critical pressure of CO2 is 7.39 MPa and the
critical temperature is 31.05 ºC: also, specify the value of Z.
A steam power plant operates with a maximum pressure of 3500 psia and maximum temperature of...
A steam power plant operates with a maximum pressure of 3500
psia and maximum temperature of 1050 oF. Assume a simple
Rankine cycle, condenser pressure is 10 psia, and turbomachinery is
isentropic.
Turbine work in Btu/lb
Pump work in Btu/lb
Heat addition in steam generator in Btu/lb
Cycle thermal efficiency
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
ADVERTISEMENT
 genius_generous answered 3 years ago
genius_generous answered 3 years ago