Question
In: Other
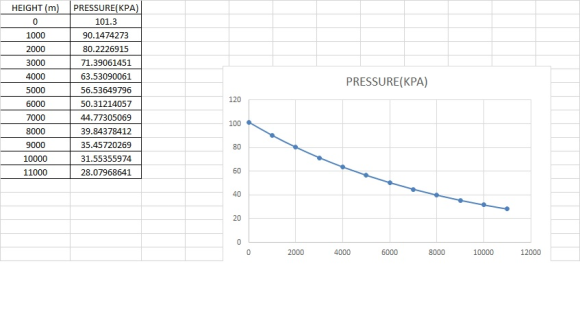
a) If the atmospheric condition at sea level is 101.3 kPa and 18oC, estimate and plot...
a) If the atmospheric condition at sea level is 101.3 kPa and 18oC, estimate and plot the pressure as a function of elevation for the following conditions:
i. Air temperature is constant
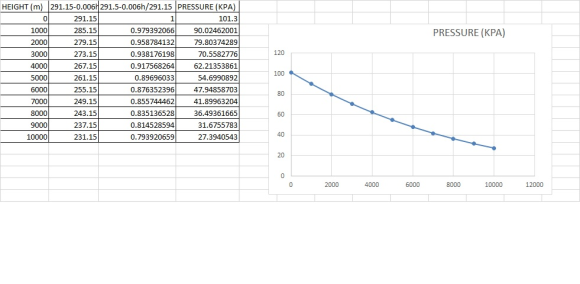
ii. Air temperature decreases linearly with elevation at a rate of 6.0oC/km.
b) Use the result above to estimate pressure and temperature of air on top of Mount Everest (height ~ 8,800 m).
State all assumptions used.
Solutions
Expert Solution
 HERE k IS
BOLTZMANCONSTANT.
HERE k IS
BOLTZMANCONSTANT.




 DEAR STUDENT, IF YOU HAVE ANY
DOUBT REGARDING ANY STEP OF THE SOLUTION KINDLY ASK ME. I SHALL TRY
MY BEST TO RESOLVE YOUR QUERY. THANK YOU.
DEAR STUDENT, IF YOU HAVE ANY
DOUBT REGARDING ANY STEP OF THE SOLUTION KINDLY ASK ME. I SHALL TRY
MY BEST TO RESOLVE YOUR QUERY. THANK YOU.
Related Solutions
1a) If the atmospheric condition at sea level is 101.3 kPa and 18oC, estimate and plot...
1a) If the atmospheric condition at sea level is 101.3 kPa and
18oC, estimate and plot the pressure as a function of elevation for
the following conditions:
i. Air temperature is constant.
ii. Air temperature decreases linearly with elevation at a rate of
6.0oC/km.
1b) Use the result above to estimate pressure and temperature of
air on top of Mount Everest (height ~ 8,800 m). State all
assumptions used.
Question 1. The height of a mercury column for atmospheric pressure at sea level is h...
Question 1. The height of a mercury column for atmospheric
pressure at sea level is h = 0.760 + or - 0.003m of mercury
(m-Hg).If the density of mercury is p = 13600 + or -
1000kg/m3 and the acceleration due to gravity, g = 9.8 +
or - 0.1ms-2 , determine the value of atmospheric
pressure Po = pgh and its uncertainty in SI units.
Question 2. A particle is moving in a straight line so that its
velocity...
At some location and at some initial time the atmospheric pressure at sea level is 1000mb...
At some location and at some initial time the atmospheric
pressure at sea level is 1000mb and the temperature between sea
level and 900mb surface is 0°C. The atmosphere is in hydrostatic
balance. (a) What is the height above sea level of the 900mb
surface? (b) Over a period of one day the temperature of sea level
and the 900mb surface rises from 0 to 3°C, but the height of
the 900mb surface does not change. What is the new...
A liquid mixture of benzene-toluene is to be distilled in a fractionating tower at 101.3 kPa...
A liquid mixture of benzene-toluene is to be distilled in a
fractionating tower at 101.3 kPa pressure. The feed of 100 kg mol/h
is liquid, containing 45 mol benzene and 55 mol% toluene, and
enters at 327.6 K. A distillate containing 95 mol benzene and 5 mol
toluene and a bottoms containing 10 mol benzene and 90 mol toluene
are to be obtained. The reflux ratio R is 4: 1. Determine the kg
moles per hour distillate, kg moles per...
A liq mixture of benzene-toluene is to be distilled in a fractionating tower at 101.3 kPa...
A liq mixture of benzene-toluene is to be distilled in a
fractionating tower at 101.3 kPa pressure. The feed of 100 kg mol/h
is liquid and it contains 45 mole % benzene and 55 mole % toluene
and enters at 327.6 K (130 ◦F). A distillate containing 95 mole %
benzene and 5 mole % toluene and a bottoms containing 10 mole %
benzene and 90 mole % toluene are to be obtained. The average heat
capacity of the feed...
What is the theoretical boiling point of 1 M NaCol at sea level (normal atmospheric pressure)?...
What is the theoretical boiling point of 1 M NaCol at sea level
(normal atmospheric pressure)? What is the freezing point of the
same solution? Show calculations.
electronic psychrometric plotting program, let’s consider atmospheric conditions at sea level with a dry bulb temperature...
electronic psychrometric plotting program, let’s consider
atmospheric conditions at sea level with a dry bulb temperature
(Tdry bulb ) of 23˚C and a relative humidity (RH) of 30%. Plot this
state point on the pyschrometric chart available in PsycPro
software. Use PsycPro to determine the following: a. Wet bulb
temperature (WBT) of the air b. Dew point temperature (DPT) of the
air c. Humidity ratio (specific humidity) d. Enthalpy e. Specific
volume of the air (i) Provide the values for...
Two flash resistance drums operated at 101.3 kPa are connected continuously. You want to separate the...
Two flash resistance drums operated at 101.3 kPa are connected
continuously.
You want to separate the hexaneooctane mixture. (Make the effective
number two digits.
(a) fed feed with a zhexane of 0.3, 900 lbmol/hr, making V/F
one-third flash at this time
How much is the liquid and vapor composition (yhexane,1, xhexane,1)
of the hexane leaving the drum?
(b) (a) where the vapor disillate obtained through the problem is
to be re-separated using the flash resistance drum;
All. The hexane composition...
1) A 2.00 g sample of gas occupies 8.40 m3 at 0°C and 101.3 kPa pressure....
1) A 2.00 g sample of gas occupies 8.40 m3 at 0°C and 101.3 kPa
pressure. Calculate the volume at 0°C and a pressure of 84.0
kPa.
2) Small amounts of hydrogen are conveniently prepared by
reacting zinc with hydrochloric acid. Zn + 2HCl --> ZnCl2 + H2
How many grams of zinc are required to prepare 2.50 L H2(g) at 765
Torr and 22°C?
An open empty tank (filled with atmospheric air at 27oC and 100 kPa) is filled with...
An open empty tank (filled with atmospheric air at 27oC and 100 kPa) is filled with liquid nitrogen as much as 14 kg quickly. After that, the tank is immediately closed and allowed to stand for a certain period of time. The tank is equipped with an open pipe manometer where the manometer fluid used is mercury (s.g. = 13.55). In the condition of the closed tank, the results of reading the manometer when it is constant shows that the...
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
ADVERTISEMENT
 Amanda K answered 3 years ago
Amanda K answered 3 years ago