Question
In: Chemistry
Draw the alcohol that is oxidized to give the product shown below. Show all hydrogen atoms.
Draw the alcohol that is oxidized to give the product shown below. Show all hydrogen atoms.
Solutions
Expert Solution
Concepts and reason
The concept used to solve this question is to draw the alcohol structure, which is to be oxidized to give the required aldehyde. The alcohols are the organic compounds that consist of at least one hydroxy (-OH) functional group. The product formed in the oxidation of alcohol depends on the type of alcohol and reagent used in the oxidation reaction. The primary alcohols on oxidation yield aldehydes, whereas secondary alcohols on oxidation yield ketone as a product.
Fundamentals
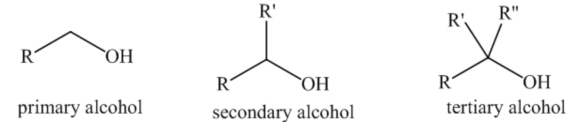
The organic compounds which contain the -OH group as a functional group are known as alcohols. The general formula for alcohol is R-OH. The alcohols can be classified as primary, secondary, and tertiary depends on the number of carbon atoms bonded to the carbon bearing the hydroxy group. The representation of the three types of alcohols is shown below.
Oxidation of alcohols:
Alcohols are the most important compounds in organic synthesis, and they involve in different kinds of reactions. The main reactions of alcohols are oxidation reactions. Alcohols undergo oxidation reactions when treated with an oxidizing agent. Primary alcohols can be oxidized to give aldehydes with mild oxidizing agents or give carboxylic acids a strong oxidizing agent. Secondary alcohols can be oxidized to give ketone as a product.
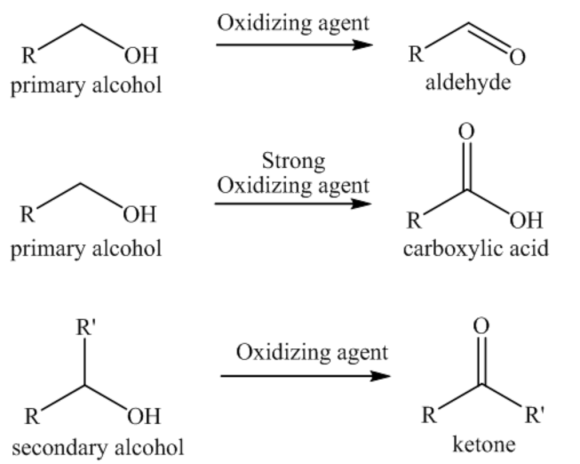
In the above oxidation reaction, one molecule of hydrogen is removed from the molecule.
The given reaction is an oxidation reaction. The structure of the product is as follows:
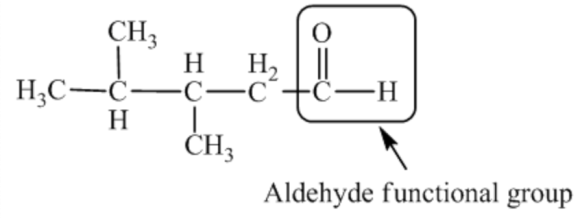
The functional group present in the above compound is aldehyde functional group \((-\mathrm{CHO})\). And, the given aldehyde is formed by the oxidation reaction.
To find out the structure of the starting alcohol that is to be oxidized, it is important to identify the functional group present in the product. By looking at the structure, it is observed that the functional group present in the product is the aldehyde functional group. The aldehyde is formed through an oxidation reaction.
The functional group present in the given product is the aldehyde functional group, and it is formed through an oxidation reaction. From the discussion in the oxidation of alcohols, aldehydes are formed by the oxidation of primary alcohols. Thus, the starting material must be primary alcohol. Therefore, the structure of the alcohol and the corresponding oxidation reaction is shown below.
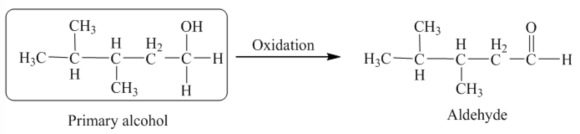
The structure of the alcohol that is to be oxidized to give the product is drawn below.
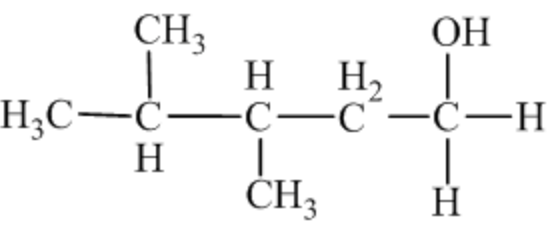
The aldehyde functional group is present in the product, and the oxidation reaction forms it. From the discussion in the oxidation of alcohols, aldehydes are formed by the oxidation of primary alcohols. Thus, the starting material must be primary alcohol. The structure of the required primary alcohol can be drawn by adding the two hydrogen atoms across the product's carbonyl bond. Therefore, the structure of the alcohol that is to be oxidized is drawn.
Related Solutions
Draw the product of the oxidation of the following aldehyde. Include all hydrogen atoms in your structure.
Draw the organic product (if any) expected from the following reaction: (include all hydrogen atoms) CH3CH2CH2OH + K2Cr2O7(aq) H2SO4
William Prout (1815) proposed that all other atoms are built up of hydrogen atoms, suggesting that all elements
Show that the statement that the Sun’s atoms are made up of 92% Hydrogen is consistent...
draw the reaction between isobutyl alcohol and propyl alcohol. give the common and IUPAC names of...
Draw the major organic product of the reaction shown below. K2Cr2O7 H2SO4, H2O
In the reaction shown below, what species is oxidized? 2NaI + Br2 → 2NaBr + I2...
1 Hydrogen and chlorine react to produce hydrogen chloride (HCl) as shown below. At equilibrium, the...
The energy level diagram of the hydrogen atom is shown the figure below
Draw the major organic product of the reaction shown below.Draw the major organic product of...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago