Question
In: Chemistry
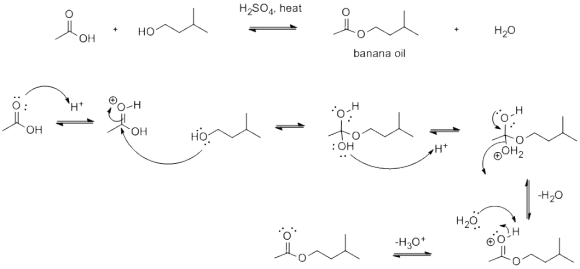
write the complete mechanism for the preparation of banana oil why do you use sulfuric acid...
write the complete mechanism for the preparation of banana oil
why do you use sulfuric acid in the reaction
explain why we used simple distillation in order to isolate our product. Describe what is actually happening in the process.
Calculate and discuss your % yield...
explain why reflux is neccesary in this experiment..
Solutions
Expert Solution
Isoamyl acetate (banana oil) is prepared by the acid catalyzed reaction (Fischer esterification) between isoamyl alcohol and glacial acetic acid as shown in the reaction equation below.
Typically, sulfuric acid is used as the catalyst for fast conversion of product from starting alcohol and acid.
The distillation is required because the side product of this reaction is water. From Le Chatelier's principle, there are two ways to adjust reagent concentrations to force isopentyl alcohol to become Isoamyl acetate (product). One way is to remove product (ie. water) as it forms and the other way is to use a large excess of acetic acid.
This experiment attempts to prepare isopentyl acetate from acetic acid and isopentyl alcohol. The reaction is catalyzed by sulfuric acid, but the catalyst affects only the rate of reaction, and not the extent of reaction. The desired product accumulates only if the equilibrium constant is favorable. The equilibrium constant for this reaction is rather small (~4). Therefore, simply mixing equal amounts of the starting materials will convert only about 67% of the starting material into product. So, in order to attain the activation energy for this conversion, reflux condition is required.
Related Solutions
1. Write the balanced equation (not the mechanism) for the protonation of nitric acid by sulfuric...
The name of the experiment is Preparation of Synthetic Banana oil. The name of the author...
1. You use an ice bath when adding nitric acid to sulfuric acid. Why? 2. You...
Fischer Esterification: Synthesis of Isoamyl Acetate (Banana Oil) 4) a) What is the role of sulfuric...
1) Draw a mechanism for the reaction of the preparation of dibromosuccinic acid and predict the...
Why is the formal charge of sulfur on sulfuric acid 0 (would be appreciated if you...
Need in JAVA. You are to use Binary Trees to do this Program. Write a complete...
This is a preparation of aspirin and oil of wintergreen lab 1. Why was cold as...
What salt is formed in the reaction of silver with sulfuric acid? Write the formula of...
Can anyone explain the complete mechanism for preparation of amines via gabriel synthesis starting with phthalimide?
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago