Question
In: Physics
determines the specific heat of the iron if 50 g of cloves are placed in 100...
determines the specific heat of the iron if 50 g of cloves are placed in 100 ml of cold water at 10º C and 100 ml of hot water at 70º C are added.
The temperature of the mixture is 35.5ºCSolutions
Expert Solution
1 L = 1000 g 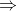 1 mL = 1 g
1 mL = 1 g 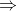 100 mL = 100 g = m ( mass of water )
100 mL = 100 g = m ( mass of water )
Mass of iron = M = 50 g
Specific heat of water = cw = 4.2 J / g oC
Final temperature of the mixture = T = 35.5o C
Let, the specific heat of iron be CI in J / g oC and initial temperature of iron be t
Hence, according to principle of calorimetry,
Heat gained by ( iron + water ) at 10 C = Heat gained by water at 70o C
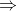 M x CI x ( T - t ) + m x cw x ( T - 10 ) = m
x cw x ( 70 - T )
M x CI x ( T - t ) + m x cw x ( T - 10 ) = m
x cw x ( 70 - T )
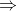 50 x CI x ( 35.5 - t ) + 100 x 4.2 x ( 35.5 - 10 ) = 100
x 4.2 x ( 70-35.5 )
50 x CI x ( 35.5 - t ) + 100 x 4.2 x ( 35.5 - 10 ) = 100
x 4.2 x ( 70-35.5 )
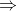 50 ( 35.5 - t ) CI + 10710 = 14490
50 ( 35.5 - t ) CI + 10710 = 14490
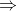 50 ( 35.5 - t ) CI = 14490 - 10710 = 3780
50 ( 35.5 - t ) CI = 14490 - 10710 = 3780
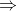 ( 35.5 - t ) CI = ( 3780 / 50 ) = 75.6
( 35.5 - t ) CI = ( 3780 / 50 ) = 75.6
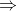 Specific heat of iron = CI = 75.6 / ( 35.5 - t ),
where, t = initial temperature of iron.
Specific heat of iron = CI = 75.6 / ( 35.5 - t ),
where, t = initial temperature of iron.
Related Solutions
a) A 500.-g iceberg at -5.00°C is placed in a 226 g beaker with specific heat...
A 62.5 g of iron with a heat capacity of .450 J/C is heated to 100...
A 100 g piece of granite and a 100 g piece of lead are placed in...
Part 1) In the laboratory a student determines the specific heat of a metal as follows:...
2. A 155 g sample of iron metal at 120.0C is placed into 250.0 g of...
Specific heat of ice: 2.09 J/(g⋅∘C Specific heat of liquid water: 4.18 J/(g⋅∘C) Enthalpy of fusion:...
Water's heat of fusion is 80. cal/g , its specific heat is 1.0calg⋅∘C, and its heat...
A 10 g object at 50oC is placed in 100 g water at 39.8oC in a...
Water's heat of fusion is 80. cal/g , and its specific heat is 1.0calg⋅∘C . Some...
1. Specific heat of water is 4.184 J/g ˚C. What is the heat in KJ gained...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
 genius_generous answered 3 years ago
genius_generous answered 3 years ago