Question
In: Mechanical Engineering
Define mass density and specific weight. Define specific volume. Define specific gravity.
- Define mass density and specific weight.
- Define specific volume.
- Define specific gravity.
Solutions
Expert Solution
ANSWER :
1) MASS
DENSITY (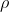 )
:
)
:
- Mass density (also known as specific mass)
is defined as it is the mass per unit volume of substance or it is
the ratio of mass of substance per unit volume. It means
that , the amount of mass (of a particular substance) contained in
a unit volume.It is denoted by the symbol
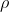 (rho).SI unit is Kg/m3. (Mass density of water is 1000 kg/m3
it means that 1000 kg mass of water contained in unit volume
)
(rho).SI unit is Kg/m3. (Mass density of water is 1000 kg/m3
it means that 1000 kg mass of water contained in unit volume
) - Specific Weight (
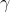 ): The specific weight is defined as it the weight of substance per
unit volume (KN /m3 ,SI unit). (for example
::The specific weight of water is (mass density x gravity) 1000
kg/m3 x 9.807 m/s2 = 9807 KN/m3)
): The specific weight is defined as it the weight of substance per
unit volume (KN /m3 ,SI unit). (for example
::The specific weight of water is (mass density x gravity) 1000
kg/m3 x 9.807 m/s2 = 9807 KN/m3)
2) SPECIFIC VOLUME :
- Specific volume is defined as it is the volume per unit mass of substance. It means that the number of cubic meters occupied by 1 Kg of particular substance.It is reciprocal of mass density . The SI unit is m3/kg.
3)SPECIFIC GRAVITY :
- Specific gravity is defined as it the ratio of density (mass per unit volume) of a substance to the density of standard substance (of a equal volume).
- Water is taken as the standard fluid for liquids and air is taken as the standard fulid for gases.
- The density of fluid can be calculated with the help of specific gravity . If we know the specific gravity of fluid then, density = specific gravity of fluid x density of water
- for example the density of meccury = specific gravity of meccury (13.6) x density of water 1000 = 13600 kg/m3
Related Solutions
Please be clear in explanation! 1. Define density, mass, weight, and specific gravity. A room is...
Please be clear in explanation!
1. Define density, mass, weight, and specific gravity. A room is
4m wide, 5m long, and 3m high. Air pressure in the room is 110 KPa
absolute and the temperature is 27C. Assuming ideal gas behaviour,
determine the mass, density, and specific gravity of the air using
water as reference density. (The density of water is 1000kgm-3, the
molar mass of air is 29g/mol, and R=8.314 J/(kg mol)).
Let the minimum fresh air requirement for...
1 a) How is density , specific gravity and specific weight related to each other? Write...
1 a) How is density , specific gravity and specific weight
related to each other? Write formula and specify SI units for each
of these terms.
1 b) How does density, vapour pressure and viscosity change with
temperature and how do you explain these changes ?
(Fluid):- Density, Specific weight, Specific gravity, Salinity, Compressibility, Vapor pressure, and Surface tension: Explain how these...
(Fluid):-
Density, Specific weight, Specific gravity, Salinity,
Compressibility, Vapor pressure, and Surface tension:
Explain how these properties might explain the behavior of a
fluid, or explain something you observe every day
please give one brief sentence for each.
does gravity influence weight? does gravity influence mass?
does gravity influence weight?
does gravity influence mass?
compare the apparent specific gravity of the glass to its unit weight. Which is greater? and...
compare the apparent specific gravity of the glass to its unit
weight. Which is greater? and why
1. Determine the volume of the aluminum foil using the density of foil. Volume = mass...
1. Determine the volume of the aluminum foil using the density
of foil.
Volume = mass (g) x density g/cm3
Volume = 4.29 g/ 2.70 g/cm3
Volume= 1.59 cm3
2. Determine the volume of one atom of aluminum.
3. Determine the number of atoms of aluminum in the foil
sample.
4. Determine the moles of aluminum.
5. Calculate Avogadro’s number.
The formula used to calculate the density of a substance is mass divided by volume. It...
The formula used to calculate the density of a substance is mass
divided by volume. It is expressed as d = m/v. Ask users
for the mass and volume of a substance and calculate the density
for them.
Output:
Enter the mass of a substance in grams (integers only): [assume
user inputs 10]
Enter the volume of a substance in milliliters (integers only):
[assume user inputs 4]
The density of your substance is 2.5
Solve in C++.
If the specific weight of a liquid is 8.1 kN/m3, what is its density? If the...
If the specific weight of a liquid is 8.1
kN/m3, what is its density?
If the specific volume of a gas is 0.70 m3/kg, what
is its specific weight in N/m3?
If a certain liquid weighs 8600 N/m3, what are the
values of its density, specific volume, and specific gravity
relative to water at 40C?
At a depth of 12 m of a solvent the pressure at the bottom of
the covered storage tank due to the solvent was 350...
17. Due to Mars having 38% less gravity than Earth, muscle mass and bone density A....
17. Due to Mars having 38% less gravity than Earth, muscle mass
and bone density
A. would increase in Martian humans
B. decrease in Martian humans
C. remain the same in Martian humans
18. During aging, the reduction of HCL by the stomach
A. causes constipation
B. increases appetite
C. reduces the adsorption of vitamin B12
D. increases the size of stomach
27. Lack of microbes on the sterile Martian surface may
A. weaken our immune system
B. amplify our...
calculate the mass concentration and molar concentration for 67.2% (by weight) nitric acid solution. The density...
calculate the
mass concentration and molar concentration for 67.2% (by weight)
nitric acid solution. The density of nitric acid solution (67.2% by
weight) is 1.4 g/cm
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
- For this assignment you will write a program with multiple functions that will generate and save...
- How many grays is this?Part A A dose of 4.7 Sv of γ rays in a...
- how to operate a business?
ADVERTISEMENT
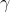 ): The specific weight is defined as it the weight of substance per
unit volume (KN /m3 ,SI unit). (for example
::The specific weight of water is (mass density x gravity) 1000
kg/m3 x 9.807 m/s2 = 9807 KN/m3)
): The specific weight is defined as it the weight of substance per
unit volume (KN /m3 ,SI unit). (for example
::The specific weight of water is (mass density x gravity) 1000
kg/m3 x 9.807 m/s2 = 9807 KN/m3) samet mamat answered 3 years ago
samet mamat answered 3 years ago