Question
In: Chemistry
In the ground-state electron configuration of Fe3+, how many unpaired electrons are present?
Part A
In the ground-state electron configuration of Fe3+, how many unpaired electrons are present?
Express your answer numerically as an integer.
Part B
Build the orbital diagram for the ion most likely formed by phosphorous.
Use the buttons at the top of the tool to add orbital's in order of increasing energy, starting at the bottom with the lowest energy orbitals. Click within an orbital to add electrons.
Solutions
Expert Solution
Concepts and reason
The electrons are filled in the orbitals according to Hund's rule. It states that the pairing of electrons takes place only after all the degenerate orbitals are half-filled. If an atom loses electrons, it gets a positive charge, and if the atom gains electrons, it gets a negative charge.
Fundamentals
The ground state is the molecule's energy state when all the electrons are present in the lowest possible molecular orbitals. In an excited state, one electron goes from lower energy orbital to higher energy molecular orbital.
\(\mathbf{A}\)
The atomic number of Fe is 26.
It loses 3 electrons to form \(\mathrm{Fe}^{3+}\) ion.
Hence, the number of electrons in \(\mathrm{Fe}^{3+}\) is 23 . Therefore, its ground state electron configuration is,
\(1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{5}\)
Write the orbital configuration for the outermost \(3 \mathrm{~d}\) orbital as follows:
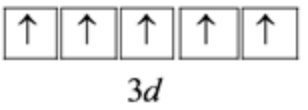
Hence, the number of unpaired electrons in \(\mathrm{Fe}^{3+}\) are 5 .
In the \(3 \mathrm{~d}\) subshell, there are 5 degenerate \(\mathrm{d}\) orbitals. Hence, according to Hund's rule, each orbital gets one electron and a total of 5 unpaired electrons.
\(\mathbf{B}\)
The most likely ion formed by phosphorous is \(\mathrm{P}^{3-}\).
The electron configuration of \(\mathrm{P}^{3-}\) is,
\(1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6}\)
The orbital diagram for \(\mathrm{P}^{3-}\) is as follows:
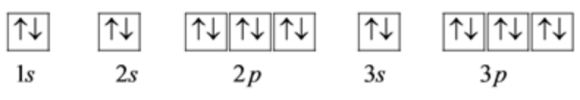
The atomic number of phosphorous is 15. Its electron configuration is,
\(1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{3}\)
It needs 3 electrons to attain an inert gas configuration.
Hence, the most likely ion formed by phosphorous is \(\mathrm{P}^{3-}\).
Related Solutions
How many unpaired electrons are present in each of the following in the ground state? O...
Based on the electron configuration and number of unpaired electrons, which are diamagnetic? Electron configuration of...
How many pairs of electrons are present in the valence shell of a neutral ground-state nitrogen...
Which element has a neon core and five valence electrons in its ground state electron configuration?
Write the Slater determinant for the ground state electron configuration of Be.
How many valence electrons are there in an element with the following electron configuration? 1s2 2s2...
The electron configuration of an atom tells us how many electrons are in each orbital. For...
Write the complete ground state electron configuration for the following (no shortcuts):
Give the electron configuration for an element with 19 electrons. How many valence electrons does this element have?
TRUE or FALSE The element with a ground state electron configuration of 1s22s22p63s2 is an alkaline...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
- For this assignment you will write a program with multiple functions that will generate and save...
- How many grays is this?Part A A dose of 4.7 Sv of γ rays in a...
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago