Question
In: Chemistry
1) The procedure stated “benzoate is protonated to make benzoic acid. Caffeine has no appreciable basicity, so...
1) The procedure stated “benzoate is protonated to make benzoic acid. Caffeine has no appreciable basicity, so it is neutral at pH 2” – what does it mean to protonate? Why would it be important to protonate benzoate? Why is caffeine not affected the same way? Cite and interact
2) UV, fluorescence and luminescence: Does Beers law hold true for all three? What dictates what kind of chemical compound will demonstrate as a UV chromophore or naturally fluoresce or naturally luminesce? Cite and interact: remember that I only want about 4-5 sentences, so be concise.
3) What do you think would have happened if we had used a diluent that was just water or was 0.010 N NaOH for this experiment? Predict what would happen to the calibration curves and the final results in each situation.
Solutions
Expert Solution
1. protonation: it means putting in an acid mean a salt. The benzoate is soluble in water, but benzoic acid does not. Caffeine can not be protonated or deprotonated. Then it will not be soluble.
2. Beer law is most suitable for uv. Other methods as fluorescence and luminescence we can use atomic or molecular spectrocopy.
Fluorescence occurs when an orbital electron of a molecule, atom, or nanostructure, relaxes to its ground state by emitting a photon from an excited singlet state:
Excitation: 
Fluorescence (emission): 
Here 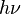 is a generic term for photon energy with h =
Planck's constant and
is a generic term for photon energy with h =
Planck's constant and 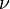 = frequency of light. The specific
frequencies of exciting and emitted light are dependent on the
particular system.
= frequency of light. The specific
frequencies of exciting and emitted light are dependent on the
particular system.
3. The experiment you did is needed.
What might have happened is related to the separation of the compound within a chemical matrix. Also the compound might have reacted with NaOH and their product is not a colored molecule. Which in the calibration curve can be seen as a non linear relation between concentration and absorbance.
Related Solutions
In order to know about solubility for caffeine and benzoic acid, I put caffeine and benzoic...
Spectrophotometric Analysis of a Mixture: Caffeine and Benzoic Acid in a Soft Drink Benzoic Acid 230nm...
Benzoic acid is a weak acid that has antimicrobial properties. Its sodium salt, sodium benzoate, is...
You need to separate a mixture of caffeine and benzoic acid. The sample has an initial...
What should the molar concentrations of benzoic acid and sodium benzoate be in a solution that...
What should the molar concentrations of benzoic acid and sodium benzoate be in a solution that...
What should the molar concentrations of benzoic acid and sodium benzoate be in a solution that...
What should the molar concentrations of benzoic acid and sodium benzoate be in a solution that...
how to make benzoic acid
What is the pH of a buffer containing 20.53g benzoic acid and 17.23g sodium benzoate in...
- In long paragraphs answer the questions below: Discuss the key components (where, when, what) and causes...
- Sinkal Co. was formed on January 1, 2018 as a wholly owned foreign subsidiary of a...
- Larry’s best friend, Garfield, owns a lasagna factory. Garfield’s financial skills are not very strong, so...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago