Question
In: Chemistry
6) What is the function of the Amberlyst 15 in the Diantilis synthesis? Briefly discuss the...
6) What is the function of the Amberlyst 15 in the Diantilis synthesis? Briefly discuss the structure of Amberlyst, why it catalyzes the reaction, and the advantage of using it. 7) In the reaction of sodium borohydride with ethyl vanillin (3-ethoxy-4-hydroxybenzaldehyde), HCl (aq) is added during the reaction workup. What is the purpose of this step? What gas is formed when HCl (g) is added? 8) What is the stoichiometry for the reaction of sodium borohydride with ethyl vanillin (look that up).
Solutions
Expert Solution
6) What is the function of the Amberlyst 15 in the Diantilis synthesis? Briefly discuss the structure of Amberlyst, why it catalyzes the reaction, and the advantage of using it
ANS) Amberlyst 15 is a one of the Ion Exchange Resin
RESIN
Resin is a sticky flammable organic substance, insoluble in water, exuded by some trees and other plants
ION EXCHANGE RESIN
An ion-exchange resin or ion-exchange polymer is an insoluble matrix normally in the form of small (0.5-1 mm diameter) beads, usually white or yellowish, fabricated from an organic polymer substrate. The beads are typically porous, providing a high surface area. The trapping of ions occurs with the accompanying releasing of other ions; thus the process is called ion-exchange. Ion-exchange resins are widely used in different separation, purification, and decontamination processes. The most common examples are water softening and water purification.
AMBERLYST 15
It is srong acedic, sulphonic acid and micrireticular polymer resin based on the crosslinked styrene divinyl benzene copolymers. Its continuous open pore structure and exelent physical, thermal and chemical stability makes it the resine of choice of many applications it also precess a greater resistance to oxidents such as chlorine, oxygen and chromates than most other polymer resins. Most of the resins are made of polystyrene sulfonate.
structure of Amberlyst 15

On other hand
The amberlyst 15 is a good catalyst Commercially available Amberlyst-15 has played an important role in organic synthesis. This review summarizes the versatile synthetic applications of Amberlyst-15 in different chemical transformations. Reactions include esterification, transesterification, Michael addition, azaMichael addition, Prins cyclization, Friedel-Crafts alkylation, acylation, metal free hydroarylation etc.
Function of the Amberlyst 15 in the Diantilis synthesis
It has the optimal balance of surface area acid capacity, activity of pore diameter to make it the choice of catalyst. One advantage is definitely to speed up chemical reactions n another is the process is less time consuming n also results in a higher yield of the product . Greater control of the rate of reaction, higher yields for reversible exothermic reactions, e.g Haber Process.
7) In the reaction of sodium borohydride with ethyl vanillin (3-ethoxy-4-hydroxybenzaldehyde), HCl (aq) is added during the reaction workup. What is the purpose of this step? What gas is formed when HCl (g) is added?
ANS) SODIUM BOROHYDRATE
It is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are used for bleaching wood pulp.[3][4] The compound is soluble in alcohols and certain ethers but reacts with water in the absence of a base.[5] NaBH4 will reduce many organic carbonyls, depending on the precise conditions. Most typically, it is used in the laboratory for converting ketones and aldehydes to alcohols. It will reduce acyl chlorides, thiol esters and imines. Under typical conditions, it will not reduce esters, amides, or carboxylic acids.[5] At room temperature, the only acid derivatives it reduces are acyl chlorides, which are exceptionally electrophilic.

Sodium borohydrate.
ETHYLVENILLINE(3-ethoxy-4-hydroxybenzaldehyde)
is the organic compound with the formula (C2H5O)(HO)C6H3CHO. This colorless solid consists of a benzene ring withhydroxyl, ethoxy, and formyl groups on the 4, 3, and 1 positions, respectively. Hydrochloric acid is a strong inorganic acid that is used in many industrial processes. The use of addition of hcl in this reaction often determines the required product quality.
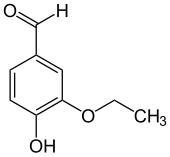
Stoichiometry is a section of chemistry that involves using relationships between reactants and/or products in a chemical reaction to determine desired quantitative data. In Greek, stoikhein means element and metron means measure, so stoichiometry literally translated means the measure of elements.
Related Solutions
Briefly discuss two mechanisms of post-translation modification of protein synthesis.
(c) What is a blending problem? Briefly discuss the objective function and constraint requirements in a...
1) Briefly explain what happens in the cellular respiration and what it function is 2) Briefly...
Please briefly discuss what is "budgetary slack" please briefly discuss what is "earnings management and possible...
Functions Create a function to calculate multiplying 15 with 6 and trace the result. Create a...
Briefly describe (in words) the regulation of ribonucleotide reductase. Briefly, how is urea synthesis regulated?
Discuss briefly what is a testable hypothesis?
Identify three major stages in protein synthesis and briefly describe what happens and the product from...
Discuss the main steps for protein synthesis
Discuss the key differences between template synthesis vs template-free synthesis
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago