Question
In: Chemistry
2. Look up a coffee-cup calorimeter in your textbook and describe the components of the apparatus....
2. Look up a coffee-cup calorimeter in your textbook and
describe the components of the apparatus. (2 points) Assuming that
the calorimeter prevents any heat to be taken in or released, the
amount of heat transferred between the system and the surroundings
should be equal in value but opposite in sign. For example, if a
hot piece of metal gives off 5 J of energy, than the surrounding
water should take in 5 J of energy. (Remember exothermic processes
have a “-“q while endothermic process have a “+” q).
qsystem = - qsurroundings
With that being said, the transfer of heat during a chemical reaction can be concluded as
qsystem (aka the reaction) = - qsurroundings (aka the solution)
and since we know that…
q = C x m x DT
we can conclude that…
crxn x mrxn x DTrxn= - (csoln x msoln x DTsoln)
(note that the “-“ sign is just to show that they are equal but opposite. The actual value of the rxn and solution depend on the reaction that took place)
Solutions
Expert Solution
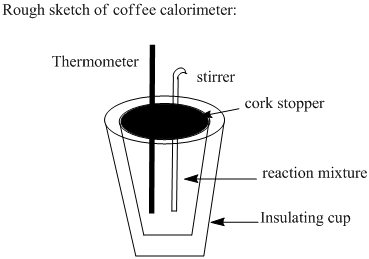
Consider mixture of reactants in aqueous solution is placed in coffee cup. As the reaction occurs it releases energy which is transferred to the calorimeter. That is, heat released by reaction will be absorbed by calorimeter and there will be temperature rise in the calorimeter.
qrxn = -(qcal) = -Ccal(change in temperature)
The reactant solutions if undergo neutralization form water, so if the heat absorbed by calorimeter and other products (excluding water) is ignored. The solution density and heat capacity can be taken just of water.
----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
calorimeter.
qrxn = -(qcal + qwater)
1) If the temperature of calorimeter (and its contents) increases, the calorimeter (and its contents) should absorb energy. And this energy is released by the reaction. Thus, the sign of qcal, qcontents is positive and the sign of qrxn is negative.
2) If the temperature of calorimeter decreases, the calorimeter (and its contents) should release energy. And this energy is absorbed by the reaction. Thus, the sign of qcal is negative and the sign of qrxn is positive
Related Solutions
In the laboratory a"coffee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the...
In a coffee-cup calorimeter experiment, if we ignored the heat lost to the Styrofoam cup and...
In the laboratory a student uses a "coffee cup" calorimeter to determine the specific heat of...
A coffee cup calorimeter contains 480.0 g of water at 25.0 oC. To it are added:...
In the following experiment, a coffee-cup calorimeter containing 100 mL of H2O is used. The initial...
In the following experiment, a coffee-cup calorimeter containing 100 mL of H2O is used. The initial...
Part A In the following experiment, a coffee-cup calorimeter containing 100 mL of H2O is used....
In the following experiment, a coffee-cup calorimeter containing 100 mL of H2O is used. The initial...
A coffee-cup (constant pressure) calorimeter is used to carry out the following reaction in an unknown...
When 28 g of calcium chloride was dissolved in 100g water in a coffee-cup calorimeter, the...
- On June 1, 2008, Coltec Industry purchased $503,000, 11% bonds, with interest payable on January 1...
- What code would I add to the following program to have it print out the runtime...
- How do you think eMarketing should fit into the overall marketing picture? And How do some...
- Tami Tyler opened Tami’s Creations, Inc., a small manufacturing company, at the beginning of the year....
- find how many times a letter appear in a string for example yellowstone letter l appear...
- Consider an object sliding down a frictionless incline. 1. Would you expect the object to move...
- explain fully why the short run atc curve is u shaped that is falls then increases...
 queen_honey_blossom answered 18 hours ago
queen_honey_blossom answered 18 hours ago