Question
In: Chemistry
Aniline hydrochloride, [C6H5NH3]Cl, is a weak acid. Its conjugate base is the weak base aniline, C6H5NH2....
Aniline hydrochloride, [C6H5NH3]Cl, is a weak acid. Its conjugate base is the weak base aniline, C6H5NH2. Ka = 2.40 X 10-5. Calculate pH at points if 50 mL of .100 aniline hydrochloride is titrated with .185 M NaOH Calculate the pH (a)before titration begins (b)at E.P (c) at the midpoint (d) after 20 mL of NaOH (e) after 30 mL of NaOH
Solutions
Expert Solution
a)Initially, we have aniline hydrochloride in the titration flask. We shall denote it by HA. HA will dissociate as follows.
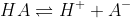
The concentrations after dissociation are as follows.
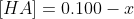
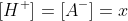
Now we calculate the concentration x using Ka.
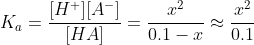
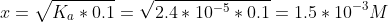
We calculate the pH using the H+ concentration calculated.
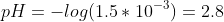
b) The equivalence volume should be calculated first.
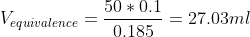
At the equivalence point, we have the conjugate base A- of the weak acid. It hydrolyses to form,
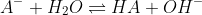
This is the hydrolysis of the conjugate base formed.
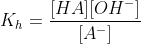
The concentration of A- at the equivalence point,
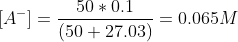
Now continuing the calculation for the concentration of OH-.
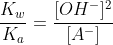
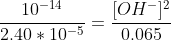
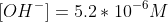
Calculate the pOH value and then the pH.
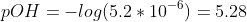
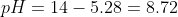
c) At the midpoint half of the HA is neutralized. Thus,
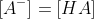
Using the Henderson-Hasselbach equation,
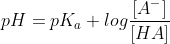
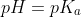
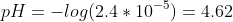
d) At 20ml of NaOH, the concentration of HA remaining is,
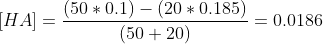
As the dissociation of HA is negligible, using henderson-hasselbach equation,
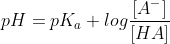
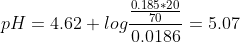
e)After 30ml of NaOH, an excess of OH- will be present in the titration flask,
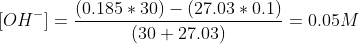
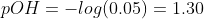
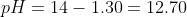
Related Solutions
Aniline hydrochloride, [C6H5NH3]Cl, is a weak acid. Its conjugate base is the weak base aniline C6H5NH2....
Write the ionization reaction of aniline, C6H5NH2, in glacial acetic acid, and identify the conjugate acid...
A buffered solution containing dissolved aniline, C6H5NH2, and aniline hydrochloride, C6H5NH3Cl, has a pH of 5.61....
A buffered solution containing dissolved aniline, C6H5NH2, and aniline hydrochloride, C6H5NH3Cl, has a pH of 5.47....
A buffered solution containing dissolved aniline, C6H5NH2, and aniline hydrochloride, C6H5NH3Cl, has a pH of 5.40....
A buffer is prepared by combining a weak acid or base with its conjugate form. A...
12. In a solution, when the concentrations of a weak acid and its conjugate base are...
Calculation of Molar Ratios of Conjugate Base to Weak Acid from pH For a weak acid...
The pH of a 0.392 M solution of the conjugate base, A-, of a weak acid,...
A buffer solution contains both a weak acid (HA) and its conjugate base (A-) so buffers...
- Aniline hydrochloride, [C6H5NH3]Cl, is a weak acid. Its conjugate base is the weak base aniline, C6H5NH2....
- Explain the RAAS by listing (and briefly describing) several steps that occur in this process. What...
- 1.What will the result of this form be? (cadr ‘(1 2 3 4 5 6)) a....
- Question 5. Why do macroeconomist require tools to bring forth policies? What are these tools? (10...
- In C++ Write an IterativeMergeSort function given these parameters and following the prompt IterativeMergeSort(vector<int> v, int...
- Current Year Preceding Year Balance Sheet: Cash $24,000 $25,000 Short-term Investments 17,000 28,000 Net Accounts Receivables...
- If the interest rate is 11%, what is the Equivalent Annual Cost (EAC) of the following...
 queen_honey_blossom answered 3 hours ago
queen_honey_blossom answered 3 hours ago