Question
In: Chemistry
Use an energy-state diagram to explain the difference between fluorescence and phosphorescence
Use an energy-state diagram to explain the difference between
fluorescence and phosphorescence
Solutions
Expert Solution
Fluorescence is a type of luminescence that emits visible light as long as there is a supply of excitation energy. In thefluorescence diagram, the excited electron jumps from level 2 (its ground state) to level 3.
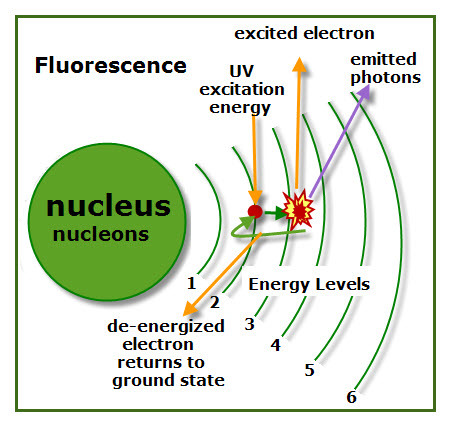
Atomic Energy Levels can be compared to stair steps. It take more energy to climb a lot of steps than just a few.
In the diagram, the excited electron on level 3 losses photons of energy and returns to its ground state. Some excited electrons jump more than one energy level. When these electrons return to their ground state, they release photons along the way as they move down the “stair steps.)
Fluorescence appears to cease the instant the excitation energy is removed, but some phosphors continue to fluoresce for a fraction of a second longer. Special instruments are needed to detect this after glow.
Example: Chemicals in the exoskeleton of a scorpion will glow as long as a black light(light that emits UVA) shines on the scorpion. Turn off the light, and the exoskeleton stops glowing. Thus, the chemicals in the scorpions exoskeleton are fluorescent.
Phosphorescent materials produce light in a
similar way as does fluorescence materials. A visible difference
between these two types of luminescence, the ability of
phosphorescencematerials to glow aft er
the excitation energy source is removed. Some
phosphorescencephosphors glow for a few minutes, while other may
glow for days.
er
the excitation energy source is removed. Some
phosphorescencephosphors glow for a few minutes, while other may
glow for days.
Example: Glow in the dark toys that when exposed to sunlight glow when placed in a dark room.
The extended after glow time forphosphorescent materials is due to the excited electrons jumping to higher energy levels than do excited electrons forfluorescence luminescence.
Excited electrons can be compared to a ball sitting on a stair step above the ground level. The higher the step the more energy needed to raise the ball to that level.
The stair steps for fluorescence can be visualized as being flat, like any steps on stairs. A ball on these flat steps could easily roll down the stairs. The same is true for excited electrons producing fluorescence luminescence.
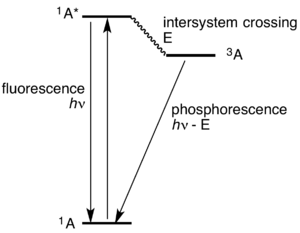
The phosphorescence diagram has stair steps with raised edges. It would be more difficult for a ball to roll down these steps. In like manner, excited electrons that producephosphorescence luminescence are trapped temporarily on each energy level. Photons continue to be emitted as the excited electron moves around until it finally has a path back to its ground state.
This is the detailed difference between fluorescence and phosphorescence and it can be simply picturised as
Related Solutions
What is the distinction between fluorescence and phosphorescence?
Which spectroscopy is more sensitive: fluorescence or absorption? Why? (2) Between fluorescence and phosphorescence: which one...
Explain why the relative intensities of the fluorescence and phosphorescence excitation spectra are the same for...
Briefly describe fluorescence using a energy level diagram.
Use the demand and supply diagram to explain the difference in pay between the doctors and...
please paraphrase this paragraph Fluorescence is a relatively faster phenomenon as compared with phosphorescence because of...
In the energy level diagram shown below, the energy difference between States 3 and 4 is...
What is the difference between a fluorescence excited spectrum and a fluorescence emission spectrum? Which one...
Describe the essential function of a fluorescence microscope and fluorescence activity using a Jablonski diagram.
a. what is the difference between a flourescence excitation spectrum and a fluorescence emission spectrum?. which...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 queen_honey_blossom answered 1 month ago
queen_honey_blossom answered 1 month ago