Question
In: Physics
A flask with a volume of 1.50L , provided with a stopcock, contains ethane gas (C2H6)...
A flask with a volume of 1.50L , provided with a stopcock, contains ethane gas (C2H6) at a temperature of 310K and atmospheric pressure 1.013
Solutions
Expert Solution
Here is what I solved before, please modify the figures as per your question. Please let me know if you have further questions. Ifthis helps then kindly rate 5-stars.
A flask with a volume of 1.50 L, providedwith a stopcock, contains ethane gas (C2H6)at 300 K and atomospheric pressure (1.013 x 105Pa). The molar mass of ethane is 30.1 g/mol. The systemis warmed to a temperature of 380 K, with the stopcock open to theatmosphere. The stopcock is then closed, and the flask cooledto its original temperature. a) What is the final pressure ofthe ethane in the flask? b) How many grams of ethane remainin the flask?
Given P1=1.013*105Pa
V1=1.5*10-3m3
T1=300K
T2=380K
We know that the ideal gas equation is 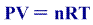
From this the pressure (P) is directly proportinal to thetemperature(T)
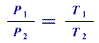
Substitute the values in this formula we get the finalpressure.
b)Given M=30.1g/mol
The mass of the Ethane is 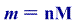
=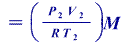
In this R is real gas constant=8.314J/mol.K
Substitute the corresponding values in this formula we get themass in grams.
Related Solutions
The gas ethane, C2H6(g), can be used in welding. When ethane is burned in oxygen, the...
A 2.20 g sample of the Ethane C2H6 gas was mixed with excess oxygen gas and...
A natural gas has the following composition: CH4 (methane) = 87 %, C2H6 (ethane) = 12...
Ethane gas (C2H6) is burned with air. The fuel flow rate is 0.1 kg/s and the...
A tank contains methane at 1000psia and 140F. Another tank of equal volume contains ethane at...
A gaseous fuel mixture contains 20.7% methane (CH4), 44.0% ethane (C2H6) and the rest propane (C3H8)...
Write and balance the equation for the complete combustion of ethane, C2H6.
A student needs to determine the volume occupied by a gas in a 125 mL flask...
A flask contains 30.5 g of oxygen gas, 45.7 g of sulfur dioxide gas and 23.9...
A sample of natural gas contains 8.24 moles of methane, 0.421 moles of ethane, and 0.116...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
 genius_generous answered 2 months ago
genius_generous answered 2 months ago