Question
In: Chemistry
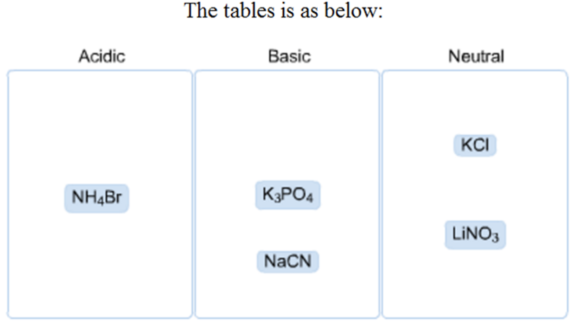
Classify these salts as acidic, basic, or neutral.
Classify these salts as acidic, basic, or neutral.
Solutions
Expert Solution
Compound: KCl
It has a cation of \(\mathrm{K}^{+}\) and \(\mathrm{a}\) anion of \(\mathrm{Cl}^{-}\)
The cation \(\mathrm{K}^{+}\) is from a strong base and the anion \(\mathrm{Cl}\) - is from strong acid.
So, it is neutral.
Compound : \(\mathrm{LiNO}_{3}\)
It has a cation of \(L i^{+}\) and an anion of \(\mathrm{NO}_{3}\)
The cation \(L i^{+}\) is from a strong base, and the anion \(\mathrm{NO}_{3}\) is from strong acid.
So, it is neutral
Compound : \(\mathrm{NH}_{4} \mathrm{Br}\)
It has a cation of \(\mathrm{NH}_{4}^{+}\) and a anion of \(\mathrm{Br}\)
The cation \(\mathrm{NH}_{4}^{+}\) is from a weak base, and the anion \(\mathrm{Br}^{-}\) is from strong acid.
So, it is acidic
Compound : \(\mathrm{K}_{3} \mathrm{PO}_{4}\)
It has a cation of \(\mathrm{K}^{+}\) and an anion of \(\mathrm{PO}_{4}\)
The cation \(K^{+}\) is from a strong base, and the anion \(\mathrm{PO}_{4}\) is from a weak acid.
So, it is basic
Compound : \(\mathrm{NaCN}\)
It has a cation of \(\mathrm{Na}^{+}\) and a anion of \(\mathrm{CN}^{-}\)
The cation \(\mathrm{Na}^{+}\) is from a strong base and the anion \(\mathrm{CN}\) ' is from a weak acid.
So, it is basic
Related Solutions
Complete the table below and classify the following salts (acidic, basic or neutral) and their pH...
Classify these amino acids as acidic, basic, neutral polar, or neutral nonpolar.
Determine if the following salts will be acidic, basic, or neutral. For each salt show the...
Classify these salts as acidic, basic, or neutral.NH4ClO4 NaF LiNO3 KCI K2CO3
Indicate if the aqueous solutions of the following salts would be acidic, basic, neutral, or “need...
Indicate if the aqueous solutions of the following salts would be acidic, basic, neutral, or “need...
Identify solutions of the following salts as acidic, basic or neutral. Identify solutions of the following...
State whether 0.1 M solutions of each of the following salts are acidic, basic, or neutral....
Determine whether aqueous solutions of the salts below are acidic (a), basic (b) or neutral (n)....
Predict whether aqueous solutions of the salts will be acidic, basic or neutral. Please explain why,...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago