Question
In: Other
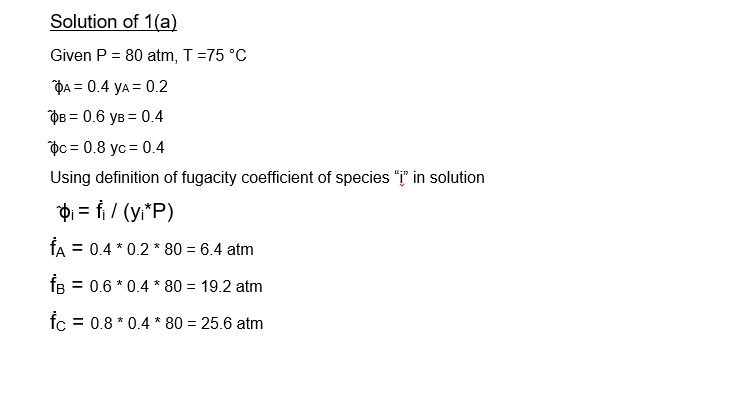
1a. A ternary mixture contains 20 mol % A , 40mol % B, and 40 mol...
1a. A ternary mixture contains 20 mol % A , 40mol % B, and 40 mol % C. At a pressure of 80 atm and a temperature of 75?C the fugacity coefficient of components A ,B and C in this mixture are 0.4, 0.6 and 0.8 respectively. What is the fugacity of the mixture?
Explain the basis of the equation you use in (a).
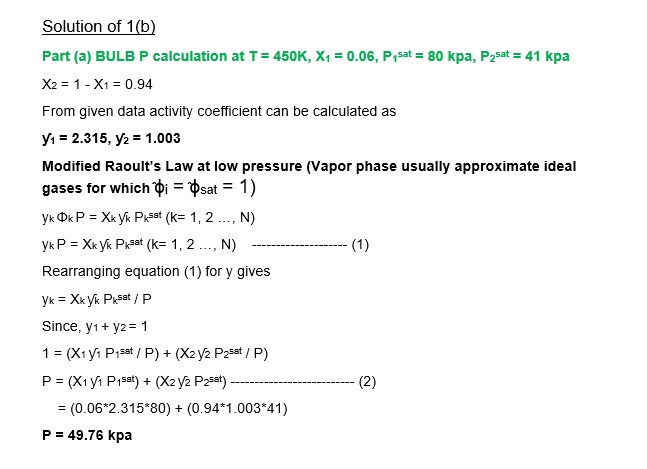
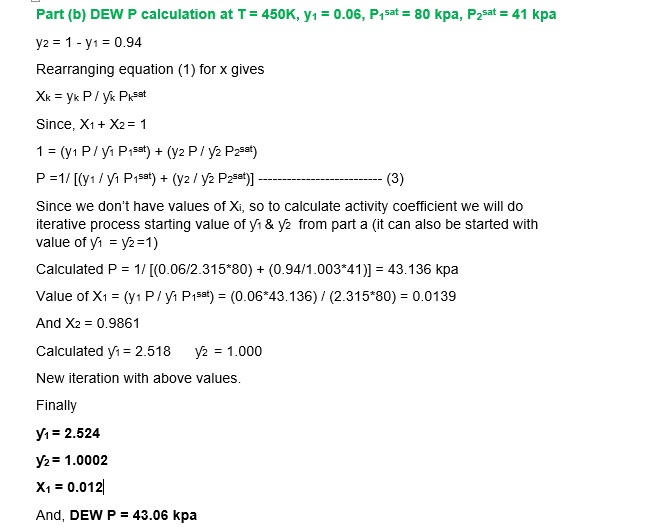
1b. For the system ethyl ethanoate (1) /n -heptane (2) at 450K , assuming the validity of the modified raoult’s law
- Make a BULB P calculation for T = 450 K, x1 = 0.06
- Make a DEW P calculation for T = 450 K, y1 = 0.06
- Determine if the system is an azeotrope
Given the following data;
Iny1 = 0.95X22 ; Iny2 = 0.95X12 P1sat 80.0 kpa ; P2sat = 41.0 kpa.
2. a. A binary mixture consists of acetonitrile (1) and nitromethane (2) which conform closely to raoult’s law. Vapor pressures for the pure species are given by the following antoine equations;
In(P1sat ,Kpa) = 2945.5t?C+224..0
In(P2sat ,Kpa) = 2972.6t?C+209.0
Determine the bubble point pressure and vapor composition for a mixture of composition X1 = 0.6 at a temperature of 65?C.
b. State the criteria for equilibrium for vapor -liquid composition.
c. Given the excess Gibb’s free energy of a solution as :
GEX1RT = B( 1-X1) find the activity coefficient expression arising out of the binary solution model .
- Show whether these expressions satisfy the Gibb’s-Duhem equation.
At 30?C and 1 atm, the volumetric data for liquid mixtures of benzene (b) and
Solutions
Expert Solution
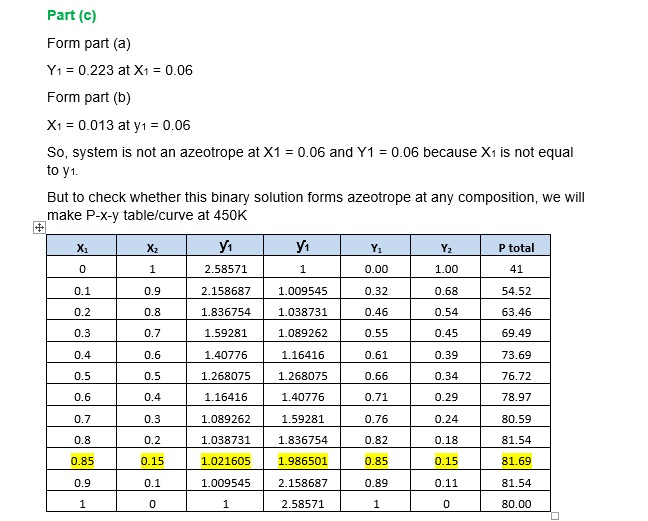
Form above table we can notice that at X1 = 0.85 = y1.
Hence, system forms Azeotrope.
Related Solutions
Suppose a ternary alloy containing 40 atomic % A, 20 atomic % B, 40 atomic %...
A gas mixture contains 0.650 mol of N2, 0.100 mol of H2, and 0.400 mol of...
1. A mixture is 15 mol% Benzene, 40 mol% Toluene and 45 mol% Xylene. Calculate the...
a. A mixture of 60 mol % n-proplycyclohexane and 40 mol % n-propylbenzene is distilled through...
We are separating a mixture that is 10 mol% methanol, 20 mol% ethanol, 30 mol% n-propanol...
A bubble-point feed mixture of 5 mol% A and 95 mol% B is to be distilled...
An equilibrium mixture is 1.0 L flask contains 0.70 mol HI(g) and 0.10 mol each of...
A mixture contains 2.3 mol of cyclohexane (C6H12) and 3.8 mol of 2-methylpentane (C6H14). At 35°C,...
A mixture of hydrocarbons (10mol% methane, 20 mol% ethane, 30mol% propane, 15mol% isobutane, 20 mol% n-butane,...
A gaseous mixture has the following composition: C₂H₄, 57% mol; Ar, 40 mol%; and He, 3%...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 Amanda K answered 3 months ago
Amanda K answered 3 months ago