Question
In: Physics
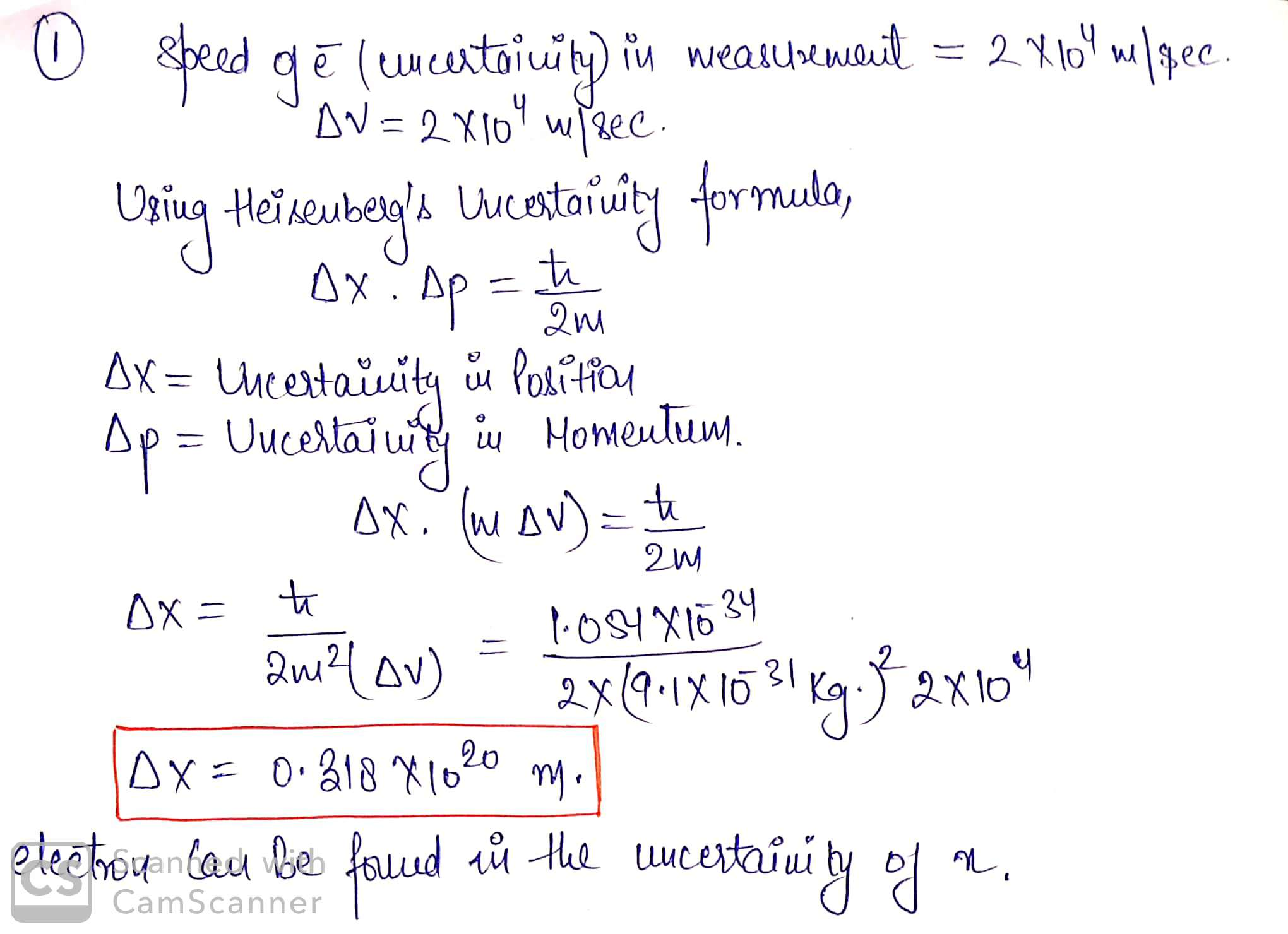
1.) The speed of an electron is measured to within an uncertainty of 2.0x104 m/s. In...
1.) The speed of an electron is measured to within an uncertainty of 2.0x104 m/s. In how large a region of space is the electron likely to be found?
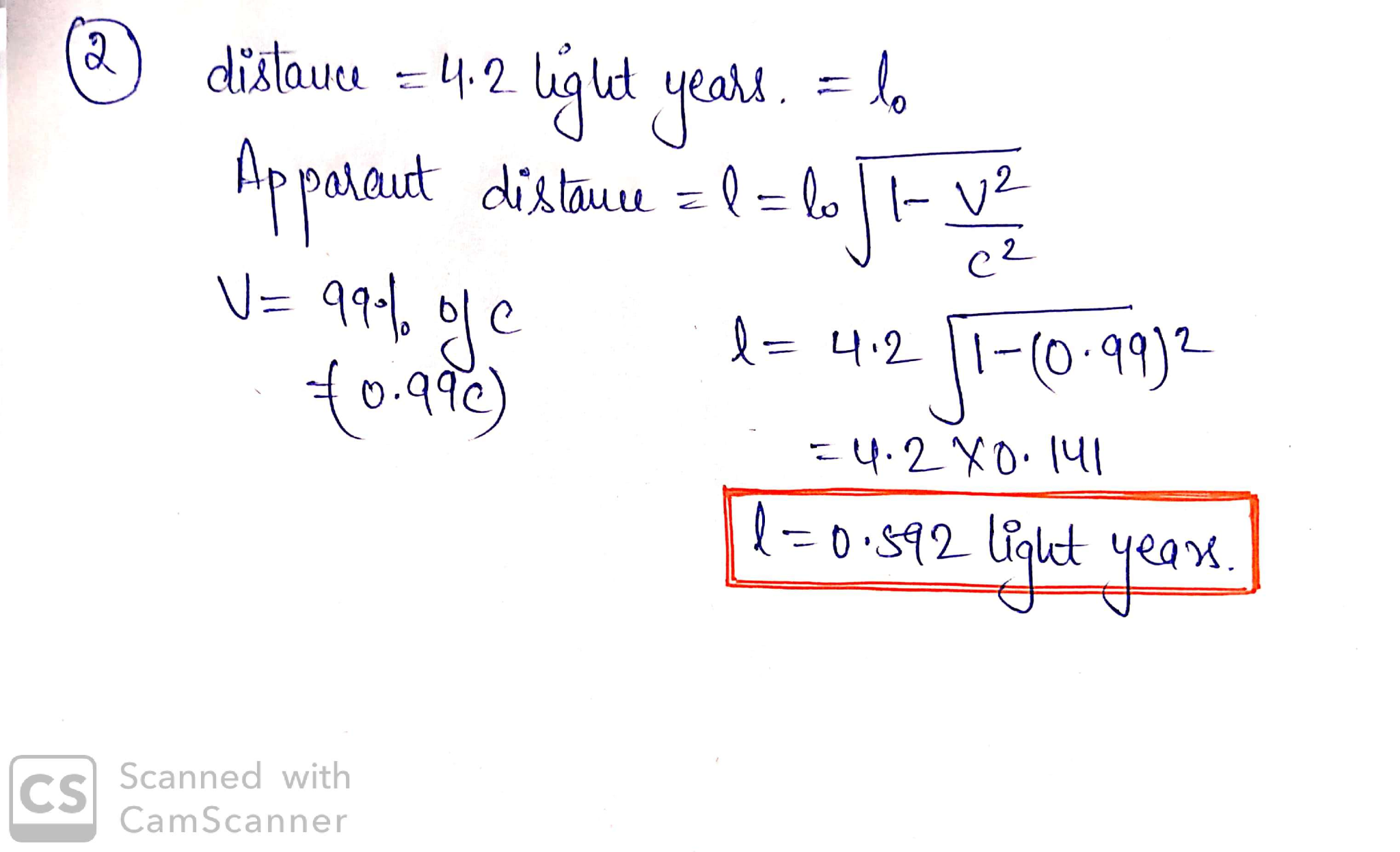
2.)The star nearest to Earth is Proxima Centauri, located at a distance of about 4.2 light years. (a) If one could travel there at 99% of the speed of light (0.99c), what would be the apparent distance to the star? (b) If you traveled to Proxmia Centauri at this high velocity, stayed there for 2 years, and then returned (also at 0.99c), how much time would have passed on Earth?
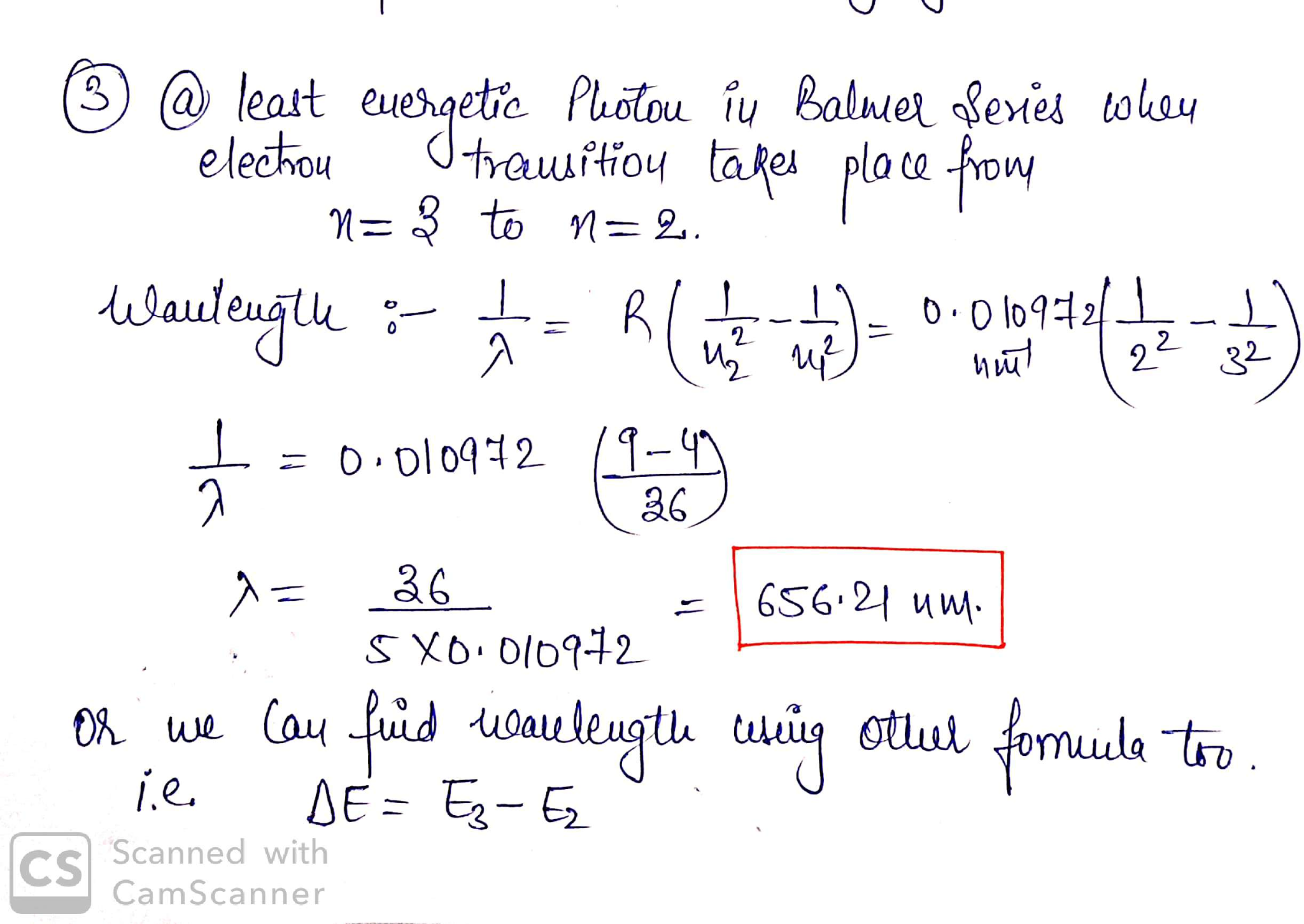
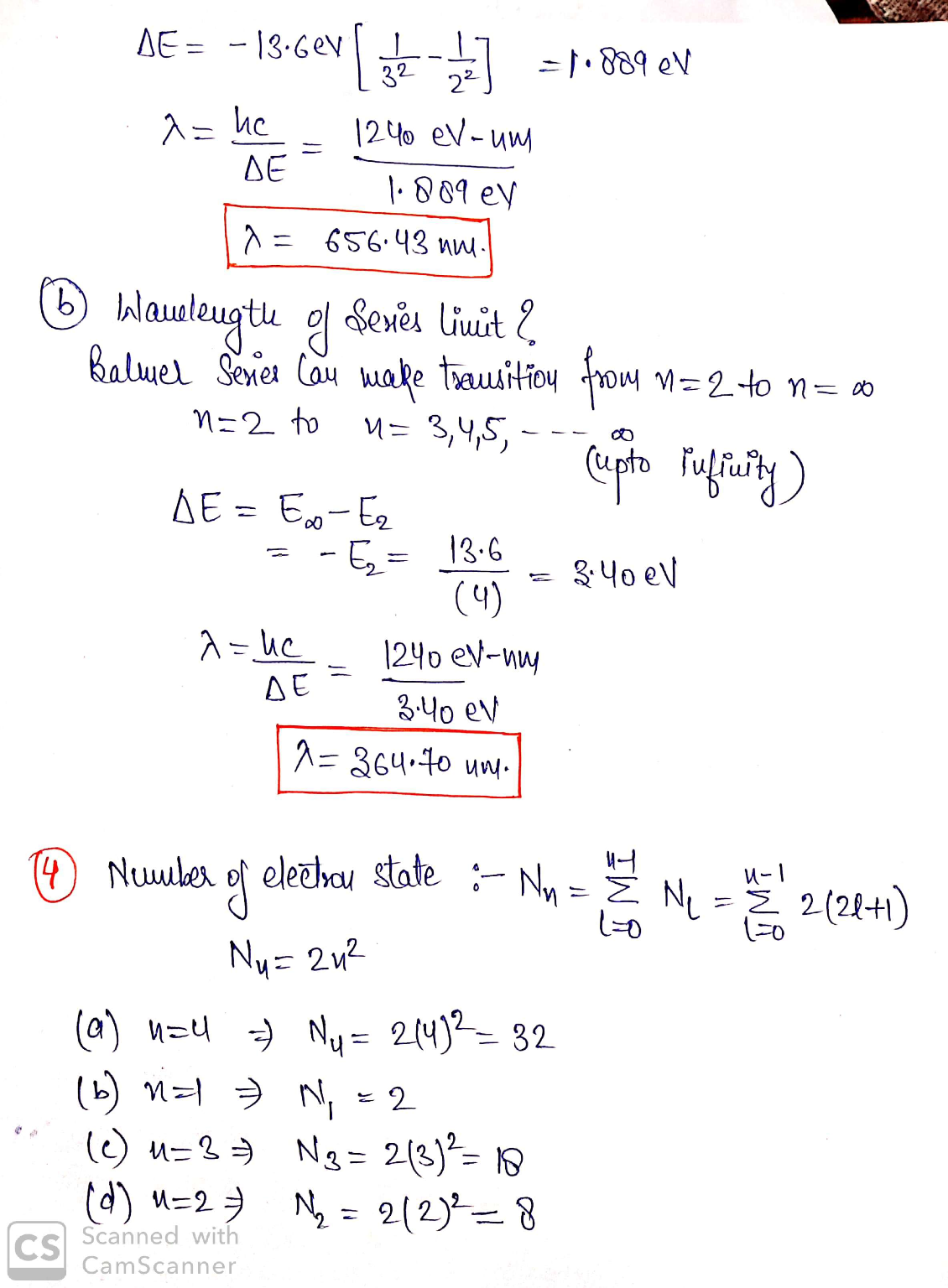
3.) (a) What is the wavelength of light for the least energetic photon emitted in the Balmer series of the hydrogen atom spectrum lines? (b) What is the wavelength of the series limit?
4.) How many electron states are there in the following shells: (a) n = 4, (b) n = 1, (c) n = 3, (d) n = 2?
5.) A sample of a certain metal has a volume of 4.0E-3 m3. The metal has a density of 9.0 g/cm3 and a molar mass of 60 g/mol. The atoms are bivalent. How many conduction electrons (or valance electrons) are in the sample?
Solutions
Related Solutions
The speed of an electron is measured to be between 4.3x10^6 m/s and 4.5x10^6 m/s. What...
Calculate the speed (in m/s) of an electron and a proton with a kinetic energy of...
The x coordinate of an electron is measured with an uncertainty of 0.200 mmmm . What...
Use the Heisenberg uncertainty principle to calculate Δx for an electron with Δv = 0.380 m/s....
an electron is moving with a speed of 2.9E5 m/s when it moves in the positive...
An electron at point A in the figure has a speed of 1.42×10^6 m/s.
An electron with an initial speed of 1.75x10^6 m/s is brought to rest by an electric...
An electron with an initial speed of 5.05x10^5 m/s is brought to rest by an electric...
An electron and a 140-g baseball are each traveling 120 m/s measured to a precision of...
An electron with a speed of 3.5 x 107 m/s travels upwards into a 3.0 T...
- In long paragraphs answer the questions below: Discuss the key components (where, when, what) and causes...
- Sinkal Co. was formed on January 1, 2018 as a wholly owned foreign subsidiary of a...
- Larry’s best friend, Garfield, owns a lasagna factory. Garfield’s financial skills are not very strong, so...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...

 genius_generous answered 4 months ago
genius_generous answered 4 months ago