Question
In: Chemistry
Identifying the highest energy form of adenosine
Identifying the highest energy form of adenosine
Select the highest energy form of adenosine from the following images.
Solutions
Expert Solution
Concepts and reason
Adenosine is a nucleoside that is made up of adenine attached to a ribose sugar molecule. Adenosine is formed in the cells through the process of metabolism in the presence of oxygen.
Fundamentals
Adenosine combines with phosphate forming molecules like ATP and ADP. These molecules help in cellular energy transfer. The molecules provide energy from the breaking of their phosphate bonds.
Adenosine phosphate or diphosphate cannot have the maximum energy as they do not contain a maximum number of phosphate groups.
The energy is released when a phosphate group is removed by breaking the phosphoanhydride bond. The number of high-energy bonds will increase with an increase in the number of phosphate groups. Hence, ATP with three phosphate groups is the highest-energy form of adenosine.
Adenosine triphosphate (ATP) is the highest-energy form of adenosine with three phosphate groups.
The highest energy form is as follows:
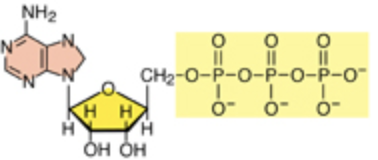
Two high-energy phosphoanhydride bonds attach the three phosphate groups. The energy is released when one phosphate group is removed from ATP by breaking the phosphoanhydride bond. And the ATP is converted to ADP. The free energy is transferred to other molecules for cellular reactions.
Related Solutions
The reaction is glucose with adenosine triphosphate (ATP) to form glucose 6- phosphate and adenosine diphosphate...
in detail how is energy stored in pyruvate converted into adenosine triphosphate (ATP) in eukaryotic cells?...
Sort the following reactions or processes based on whether they use energy from adenosine triphosphate (ATP),...
write the quantum numbers for the electrons in the highest energy level of the atom? then...
how many electrons are in the highest energy orbital of the element copper?
ADP is adenosine diphosphate. How many high-energy linkages (or bonds) does ADP contain? How do you...
Discuss the energy balance of the Earth as a whole: What form of energy does it...
Which of the following molecules contains least energy? (hint: which bond has highest energy? Which molecule...
Show the obrital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top
What is the change in the form of energy that is shown by the change in...
- C PROGRAMMIMG I want to check if my 2 input is a number or not all...
- In long paragraphs answer the questions below: Discuss the key components (where, when, what) and causes...
- Sinkal Co. was formed on January 1, 2018 as a wholly owned foreign subsidiary of a...
- Larry’s best friend, Garfield, owns a lasagna factory. Garfield’s financial skills are not very strong, so...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago