Question
In: Chemistry
Complete the mechanism for the base-catalyzed opening of the epoxide by adding any missing atoms, bonds, charges, nonbonding electron pairs, and curved arrows.
Complete the mechanism for the base-catalyzed opening of the epoxide by adding any missing atoms, bonds, charges, nonbonding electron pairs, and curved arrows.
Solutions
Expert Solution
Opening of unsymmetrical epoxide is a regioselective reaction. The opening of unsymmetrical epoxide is depended upon the reaction medium i.e. either in acidic medium or basic medium.
In an acidic medium, the opening of epoxide takes place at the sterically more crowded side because the first step is the protonation of epoxide oxygen. Then it opens at which side depends upon the stability of carbocation formed. Always the sterically more crowded side opens.
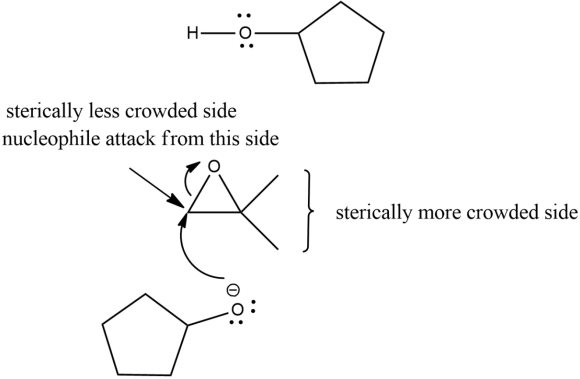
In a basic medium, the opening of epoxide is always at a sterically less crowded side takes place. Because the protonation at epoxide oxygen does not take place.
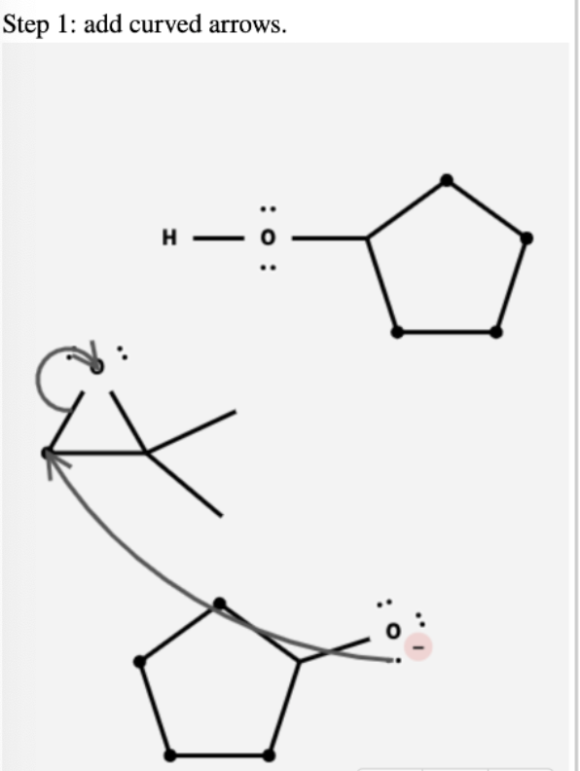
Step 1: In this step, the nucleophile opens the epoxide from the sterically less crowded side.
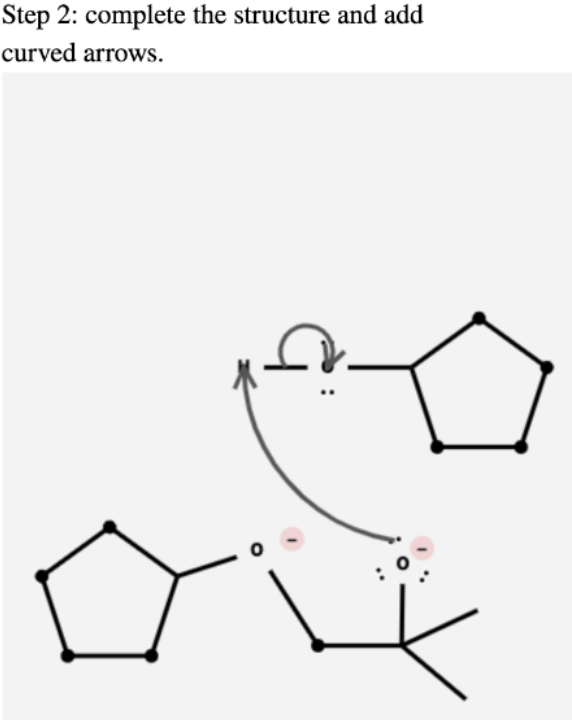
Step 2: In this step the protonation of oxygen takes place. The alcohols are good proton donors. So, the oxygen abstracts hydrogen from the cyclopentanone.
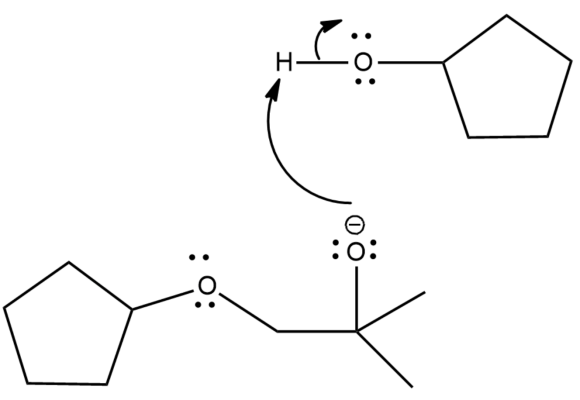
Step 3: In this step, the epoxide is converted into alcohol.
Related Solutions
Use curved arrows to predict the complete mechanism of formation of the imine shown below.
Complete the mechanism of the following Diels-Alder reaction by drawing the curved arrows for the concerted...
Draw the Lewis structure of H2O. Include any nonbonding electron pairs. Draw the molecule by placing...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago