Question
In: Chemistry
Propose a simple method for separating a mixture of a solid consisting of Al(OH)3 and Fe(OH)3....
Solutions
Expert Solution
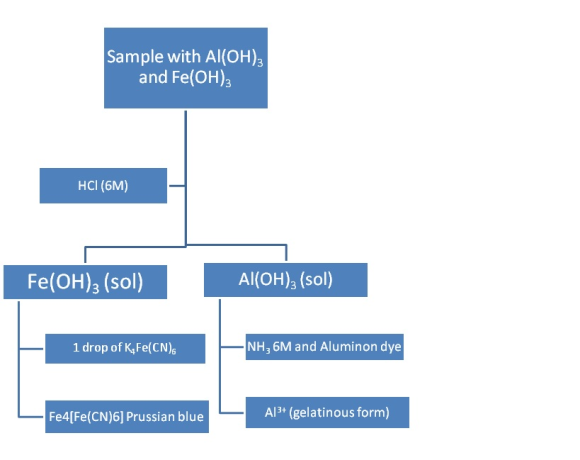
I will put the procedure to separate and identify each ion, and at the end I’ll draw the flowchart:
Before you can develop a flow scheme for analyzing an unknown, you need to know how each of the four ions to be tested (Al3+, Fe3+) will react with each of the three reagents to be used (6 M HCl, 6 M NH3, and 6 M NaOH).
Step 1. Prepare a set of 2 test tubes, each test tube containing a few drops of one of the four ions to be tested.
Step 2. Add one drop of one of the reagents, 6 M NaOH, for example, to each of the four test tubes. Observe the result. If no precipitate has formed, add 4-5 more drops of the reagent. This reagent (NaOH, HCl, or NH3) will lead to precipitates, such as Fe(OH)3, with some or all of the ions.
Fe3+(aq) + 3 OH- (aq) ? Fe(OH)3(s)
Step 3. If an ion has formed a precipitate with the reagent added in Step 2, you have to know what will dissolve this precipitate. Therefore, you should centrifuge to collect the precipitate, decant the supernatant liquid, and wash the precipitate with a few drops of distilled water. (NOTE: If no precipitate has formed, you may proceed to another ion.)
Step 4. Now you want to see what reagent will dissolve the precipitate formed in Step 2 (and washed in Step 3). Because there are three reagents that can possibly dissolve the precipitate, you will need 3 test tubes, each containing some of the precipitate. Therefore, if you have not already done so, prepare 3 batches of washed precipitate as described in Steps 2 and 3.
As we know, we already have the compounds as OH so Step 1 and 2, can be omitted.
Step 5. To each of the three test tubes containing one of the precipitates from Steps 3 and 4, say Fe(OH)3(s), add a few drops of one of the three reagents. For example, to test tube 1 add 6 M HCl, to test tube 2 add 6 M NH3, and to test tube 3 add 6 M NaOH. Some or all of the added reagents will dissolve the Fe(OH)3. You should try as hard as possible to dissolve each precipitate. It may take excess reagent or heating. Remember to stir thoroughly in any event.
CONFIRMATORY TESTS
After you have carried out the separation of a mixture of ions in solution, you must be able to confirm the presence or absence of a single ion in a solution. To do this, you must carry out confirmatory tests on individual ions. As you carry out the tests outlined below, be sure to record your observations in your notebook.
Test for aluminum, Al3+. Using a few drops of the solution containing Al3+, make it distinctly acidic with 6 M HCl. Add 1 drop of aluminon dye, and then add 6 M NH3 dropwise until the solution is basic to litmus paper. (Avoid adding an excess of NH3.) If present, Al3+ will form a gelatinous precipitate of Al(OH)3 that absorbs the red dye to give what is commonly called an "aluminum lake."
Test for iron(III), Fe3+. Make the solution acidic with 6 M HCl. Add 1 drop of K4Fe(CN)6 solution. A blue precipitate of Fe4[Fe(CN)6] (usually called "Prussian blue") confirms the presence of iron(III).
The flow chart:
Related Solutions
why is H2O2 added to the precipitate of Fe(OH)3 and Cr(OH)3?
The Ksp of Al(OH)3 is 1.0 x 10-33. What is the solubility of Al(OH)3 in 0.0010...
Below are some thermochemical data for the dissolution equilibrium of solid aluminum hydroxide in water: Al(OH)3(s)...
Balance redox reaction in basic solution: Fe(OH)2(s) + MnO4-(aq) -> MnO2 (s) + Fe(OH)3
___ FeCl3 (aq) + ___ NaOH (aq) --> Fe(OH)3 (___) + ___ NaCl (___) Balance the...
Use the reaction, 8 Al + 3 Fe3O4 = 4 Al2O3 + 9 Fe
What is the Ksp in terms of the molar solubility s for the substance Fe (OH)3?...
A white powder, consisting of a simple mixture of tartaric acid (C4H6O6) and citric acid (C6H8O7)...
FeCl3(aq) + 3 NaOH (aq) ---- Fe(OH)3(s) + 3 NaCl(aq) Is this a redox reaction in...
show the steps to balance the following equations Fe2(SO4)3 + KOH = K2SO4 + Fe(OH)3
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
 queen_honey_blossom answered 1 year ago
queen_honey_blossom answered 1 year ago