Question
In: Physics
A) what assumptions characterize an ideal gas? B)under what conditions are these assumptions most appropriate? C)...
Solutions
Expert Solution
A. The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that
(1) the gas consists of a large number of molecules, which are in random motion and obey Newton's laws of motion;
(2) the volume of the molecules is negligibly small compared to the volume occupied by the gas; and
(3) no forces act on the molecules except during elastic collisions of negligible duration.
B. Although no gas has these properties, the behaviour of real gases is described quite closely by the ideal gas law at sufficiently high temperatures and low pressures, when relatively large distances between molecules and their high speeds overcome any interaction.
C.PV=NRT in which R is called the universal gas constant. This constant has been measured for various gases under nearly ideal conditions of high temperatures and low pressures, and it is found to have the same value for all gases: R = 8.314 joules per gram-mole-kelvin.
D. 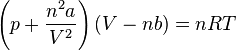
E.
In physics, pressure, P, is the measure of the force exerted over a certain area. We generally say something exerts a lot of pressure on an object if it exerts a great amount of force on that object, and if that force is exerted over a small area. Mathematically:

Pressure is measured in units of pascals (Pa), where 1 Pa = 1 N/m2.
Pressure comes into play whenever force is exerted on a certain area, but it plays a particularly important role with regard to gases. The kinetic theory tells us that gas molecules obey Newton’s Laws: they travel with a constant velocity until they collide, exerting a force on the object with which they collide. If we imagine gas molecules in a closed container, the molecules will collide with the walls of the container with some frequency, each time exerting a small force on the walls of the container. The more frequently these molecules collide with the walls of the container, the greater the net force and hence the greater the pressure they exert on the walls of the container.Doubling the no of moles of a gas doubles the collission hence doubles the pressure.
f. doubling the temperature increases the speed of collission hence doubles the pressure.
Related Solutions
Discuss under what set of conditions a real gas is most likely to deviate from ideal...
1. What is meant by an ideal gas? Under what conditions does a real gas behave...
a. under what conditions would it be appropriate to use a process costing system b. in...
There are several models derived from first principles to describe gas diffusion under ideal conditions and...
For the ideal gas law to be most applicable, it is best for the gas to...
Under which conditions or in which situations are in-depth interviews more appropriate, and under which conditions...
13-A contribution price, one lower than the regular price is most appropriate under the following conditions...
1mol of an ideal gas is inside a cylinder with a piston under a pressure of...
During an adiabatic expansion of an ideal gas, which stays constant? a) U b) H c)...
(a) What is the maximum temperature of the gas? (b) What would be the efficiency of an ideal engine with reservoirs
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 genius_generous answered 1 year ago
genius_generous answered 1 year ago