Question
In: Chemistry
Use the data provided to calculate benzaldehyde’s heat of vaporization. Vapor Pressure (torr) Temperature (K) 32.5...
Use the data provided to calculate benzaldehyde’s heat of
vaporization.
| Vapor Pressure (torr) | Temperature (K) |
| 32.5 | 363.0 |
| 934 | 458.0 |
Solutions
Expert Solution
Answer =
Heat of vaporisation of benzaldehyde = 48.98 kj/mol
---------------------------------------------------------------------------------------------------------------------------------------------------
The clausius-clapyeron is employed to calculte the heat of vapourisation of given liquid benzaldehyde.
If the vapor pressures are known at respective temperatures then the clausius-clapyeron equation detrmines the enthalpy of vaporization of the substance.
The clausius-clapyeron Equation,

P1 = 32,5 torr at T1 = 363.0 K
P2= 934 torr at T2= 458.0 K
Enthalpy of vaporisation ,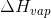 = ?
= ?
Gas constant, R = 8.3145 J mol-1 K-1

The pressure units given in Torr can be used as it is without botheration of converting to atm or pa. Since the mupltiplication facto is common for both numerator and denominator it cancels out.







= 48,982.45 j/mol
= 48.98 kj/mol [ All units balanced . Fulfilled dimension analysis]
Related Solutions
Vapor Pressure and Heat of Vaporization a. The vapor pressure of acetone at 20° C is...
The vapor pressure of water at blood temperature is 47 Torr.
At 35ºC, the vapor pressure of pure water is 42.2 torr. Calculate the vapor pressure of...
Given that the vapor pressure of water is 17.54 Torr at 20 °C, calculate the vapor-pressure...
Given that the vapor pressure of water is 17.54 Torr at 20 °C, calculate the vapor-pressure...
A certain substance has a heat vaporization of 36.63kJ/mol. At which Kelvin temperature will the vapor...
Pure liquid water has a heat of vaporization of x kJ/mol. The vapor pressure of water...
At 85oC, the vapor pressure of pure o-xylene is 55 Torr and the vapor pressure of...
Given that the vapor pressure of water is 17.54 Torr at 20 degrees celcius, calculate the...
Cyclohexane (C6H12) has a vapor pressure of 99.0 torr at 25°C. What is the vapor pressure...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago