Question
In: Chemistry
Calculate the amount (in millimoles) of protonated and unprotonated HEPES in 25 mL of 50 mM HEPES at pH 7.7 (the pKa of HEPES is 7.47)
Part 1
Calculate the amount (in millimoles) of protonated and unprotonated HEPES in 25 mL of 50 mM HEPES at pH 7.7 (the pKa of HEPES is 7.47)
Part 2
To adjust the pH of the HEPES solution in problem 7, 1mL of 50mM NaOH was added. Calculate the amount (in millimoles) of protonated and unprotonated HEPES. What is the pH after the addition of NaOH?
Solutions
Expert Solution
Hi, look at the structure of HEPES
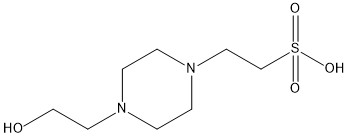
Since, we are given pKa 7.47, nitrogen is not protonated at pH values around it. We are talking about the deprotonation of sulphonic acid. Now we have gone through the basics. Consider the protonated form HL and deprotonated form L-. Consider equilibrium:
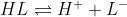 ,
we will use the Henderson-Hasselbalch equation to solve it
,
we will use the Henderson-Hasselbalch equation to solve it
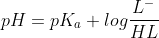
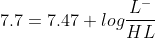
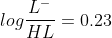
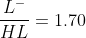
Now total millimoles are 0.05 M x 25 mL = 1.25 mmol
we can write 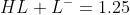 , solving these two equations will give us
, solving these two equations will give us
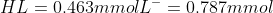
Now coming to the part two, 1 mL of 50 mM NaOH has the following millimoles of NaOH
1 mL x 0.05 M = 0.05 mmol
since the base will react with the protonated form, we will subtract these millimoles from HL and add to the L-.
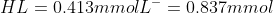
Now we can put these values in the Henderson-Hasselbalch equation again (Ignore the volume change, since it is the same for both forms so we don't need to include it in the equation)
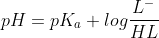
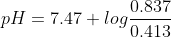 so we get the pH value 7.78. If you need any other information,
please do not hesitate to ask.
so we get the pH value 7.78. If you need any other information,
please do not hesitate to ask.
Related Solutions
You prepare 50 mL of 50 mM HEPES buffer at pH 7.4. Indicate the amounts of...
pka is 7.21, Assigned pH: ____6.8__________ . prepare 50 mL of a 50 mM buffer at...
1.Calculate the pH of a solution obtained by mixing 100 ml of 1 M HEPES and...
calculate the pH of the following aqueous solution at 25 degreesCelsius and .35M NaF (pKa for...
Calculate the pH of a solution starting with 200.0 mL of 0.010 M butanoic acid (pKa...
Calculate the pH after adding 10.00 mL of 0.15 M HCl to 50 mL of the...
You must make a sodium phosphate buffer (500 ml at 50 mM) at a pH value...
If you have 120. mL of a 0.100 M HEPES buffer at pH 7.55 and you...
You have 200 ml of 0.1M HEPES buffer, pH 7.5. You add 2 ml of 0.4M...
Calculate the pH at 25°C of 176.0 mL of a buffer solution that is 0.210 M...
- Sinkal Co. was formed on January 1, 2018 as a wholly owned foreign subsidiary of a...
- Larry’s best friend, Garfield, owns a lasagna factory. Garfield’s financial skills are not very strong, so...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago