Question
In: Chemistry
1. A) The freezing point of benzene, C6H6, is 5.500 °C at 1 atmosphere. Kf(benzene) =...
1. A)
The freezing point of benzene,
C6H6, is 5.500
°C at 1 atmosphere.
Kf(benzene) = 5.12
°C/m
In a laboratory experiment, students synthesized a new compound and
found that when 11.43 grams of the compound were
dissolved in 267.0 grams of
benzene, the solution began to freeze at
4.740 °C. The compound was also found to be
nonvolatile and a non-electrolyte.
What is the molecular weight they determined for this
compound?
____g/mol
B) The boiling point of benzene,
C6H6, is
80.100 °C at 1 atmosphere.
Kb(benzene) = 2.53
°C/m
In a laboratory experiment, students synthesized a new compound and
found that when 10.44 grams of the compound were
dissolved in294.0 grams of
benzene, the solution began to boil at
80.412 °C. The compound was also found to be
nonvolatile and a non-electrolyte.
What is the molecular weight they determined for this compound
?
____g/mol
Solutions
Expert Solution
1. A)
The freezing point of Benzene = 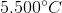
Freezing point of the solution = 
hence, the depression in freezing point observed is

The depression in freezing point is related to the molality of the solution as follows:

Where  is the proportionality constant for benzene which equals
is the proportionality constant for benzene which equals 
m is the molality of the solute.
Hence, the molality m is

molality is defiene as moles of solute per kg of solvent.
Hence, 0.148 mol of solute will be in 1k g of benzene.
weight of Benzen here = 267.0 gm = 0.267 kg
Hence, number of moles of solute present in 0.267 k of Benzene is

Amount of the compound that was added = 11.43 gm
number of moles in 11.43 gm is 0.0395 mol.
hence, molar mass of the solute is

B)
The boiling point of Benzene = 
Boiling point of the solution = 
hence, the elevation in boiling point observed is

The elevation in boiling point is related to the molality of the solution as follows:

Where  is the proportionality constant for benzene which equals
is the proportionality constant for benzene which equals 
m is the molality of the solute.
Hence, the molality m is

molality is defiene as moles of solute per kg of solvent.
Hence, 0.123 mol of solute will be in 1k g of benzene.
weight of Benzen here = 294.0 gm = 0.294 kg
Hence, number of moles of solute present in 0.294 k of Benzene is

Amount of the compound that was added = 10.44 gm
number of moles in 10.44 gm is 0.0361 mol.
hence, molar mass of the solute is

Related Solutions
The freezing point of water, H2O, is 0.000 °C at 1 atmosphere. Kf(water) = -1.86 °C/m...
The freezing point of benzene is 5.5°C. What is the freezing point of a solution of...
. The freezing point of benzene is 5.5oC What is the freezing point of a solution...
Cyclohexane has a freezing point of 6.50 ∘C and a Kf of 20.0 ∘C/m. What is...
The freezing point of water H2O is 0.00°C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves...
The freezing point of ethanol CH3CH2OH is -117.30°C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves...
A. The freezing point of water H2O is 0.00°C at 1 atmosphere. How many grams of...
The freezing point of water is 0.00°C at 1 atmosphere. If 13.93 grams of manganese(II) bromide,...
Within 100 g of pure benzene (C6H6) with freezing and boiling temperatures of 5.5 oC and...
calculate the freezing point of water (Kf= 1.86 C/m) for an ideal solution of sodium phosphate...
- Sinkal Co. was formed on January 1, 2018 as a wholly owned foreign subsidiary of a...
- Larry’s best friend, Garfield, owns a lasagna factory. Garfield’s financial skills are not very strong, so...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago